miR-1, miR-133a, miR-29b and skeletal muscle fibrosis in chronic limb-threatening ischaemia
- PMID: 39592654
- PMCID: PMC11599917
- DOI: 10.1038/s41598-024-76415-9
miR-1, miR-133a, miR-29b and skeletal muscle fibrosis in chronic limb-threatening ischaemia
Abstract
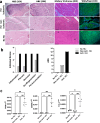
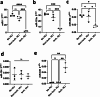
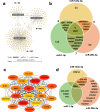
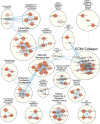
Chronic limb-threatening ischaemia (CLTI), the most severe manifestation of peripheral arterial disease (PAD), is associated with a poor prognosis and high amputation rates. Despite novel therapeutic approaches being investigated, no significant clinical benefits have been observed yet. Understanding the molecular pathways of skeletal muscle dysfunction in CLTI is crucial for designing successful treatments. This study aimed to identify miRNAs dysregulated in muscle biopsies from PAD cohorts. Using MIcroRNA ENrichment TURned NETwork (MIENTURNET) on a publicly accessible RNA-sequencing dataset of PAD cohorts, we identified a list of miRNAs that were over-represented among the upregulated differentially expressed genes (DEGs) in CLTI. Next, we validated the altered expression of these miRNAs and their targets in mice with hindlimb ischaemia (HLI). Our results showed a significant downregulation of miR-1, miR-133a, and miR-29b levels in the ischaemic limbs versus the contralateral non-ischaemic limb. A miRNA target protein-protein interaction network identified extracellular matrix components, including collagen-1a1, -3a1, and -4a1, fibronectin-1, fibrin-1, matrix metalloproteinase-2 and -14, and Sparc, which were upregulated in the ischaemic muscle of mice. This is the first study to identify miR-1, miR-133a, and miR-29b as potential contributors to fibrosis and vascular pathology in CLTI muscle, which supports their potential as novel therapeutic agents for this condition.
Keywords: Chronic limb-threatening ischaemia; Fibrosis; MicroRNAs; Muscle regeneration.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TOB is a founder, director, and equity holder in Orbsen Therapeutics Ltd. The other authors do not have competing interests to declare. Ethical approval: All animal experiments were carried out in compliance with the Directive 2010/63/EU. Ethical approval was granted by the Animal Care Research Ethics Committee (ACREC) at the University of Galway (Ireland) and appropriate individual and project authorizations were granted by the Health Products Regulatory Authority in Ireland (AE19125 /P076).
Figures



References
-
- Norgren, L. et al. Inter-society Consensus for the management of peripheral arterial disease (TASC II). in 33 Suppl 1 S1–75 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

