Carob fruit extract as naturally products corrosion inhibitor for copper-nickel alloys in brine solutions
- PMID: 39592690
- PMCID: PMC11599866
- DOI: 10.1038/s41598-024-80589-7
Carob fruit extract as naturally products corrosion inhibitor for copper-nickel alloys in brine solutions
Abstract
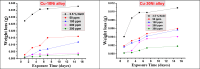
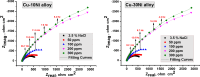
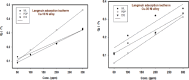
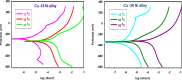
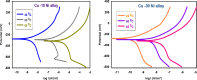
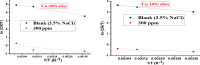
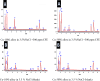
Copper-nickel alloys are the preferred material for desalination facilities and condensers and heat exchangers that use saltwater as a coolant. The eco-friendly compounds especially Carob fruit extract (CFE) has emerged as excessive green corrosion inhibitor for alloys. Cu-Ni alloys are widely used in various industries due to their excellent corrosion resistance. However, their performance can be compromised in aggressive environments like seawater (which is approximately 3.5% NaCl). To evaluate the corrosion behavior of these alloys and the effectiveness of corrosion inhibitors, researchers often employ weight loss, potentiodynamic polarization, and impedance spectroscopy techniques. The results showed that CFE exhibited a good ability to decrease the CR of alloys in 3.5% NaCl solution. The inhibition efficacy (IE) was reached to 92.6% and ̴ 83.2% at 300 ppm dose of CFE for Cu-10Ni alloy and Cu-30Ni alloy, respectively. The CR increases with temperature rising, but the addition of CFE reduces the CR, and the reduction depends on the dose of the extract. Adsorption of the extract gives a good fit to Langmuir, Temkin, and Freundlich isotherms model. The free adsorption energies of CFE on Cu-10Ni and Cu-30Ni alloys were 17.61 and 15.86 kJ mol-1, respectively, suggesting that CFE was weakly held to both alloys. The presence of a protective film on the alloys surface is confirmed by using scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectroscopy and atomic force microscope (AFM). The study suggests that utilizing affordable, natural substances as green corrosion inhibitors presents a new strategy for promoting both resource efficiency and environmental sustainability.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Hu, S., Liu, L., Cui, Y., Li, Y. & Wang, F. Influence of hydrostatic pressure on the corrosion behavior of 90/10 copper-nickel alloy tube under alternating dry and wet condition. Corros. Sci.146, 202–212 (2019).
-
- Arjmand, F. & Adriaens, A. Influence of pH and chloride concentration on the corrosion behavior of unalloyed copper in NaCl solution: a comparative study between the micro and macro scales. Mater. (Basel). 5, 2439–2464 (2012).
-
- Duran, B., Bereket, G. & Duran, M. Electrochemical synthesis and characterization of poly (m-phenylenediamine) films on copper for corrosion protection. Prog Org. Coat.73, 162–168 (2012).
-
- Sherif, M. E. S., Almajid, A. A., Bairamov, A. K. & Al-Zahrani, E. A comparative study on the corrosion of monel-400 in aerated and deaerated arabian gulf water and 3.5% sodium chloride solutions. Int. J. Electrochem. Sci.7, 2796–2810 (2012).
-
- Gaber, G. A., Maamoun, M. A. & Ghanem, W. A. Evaluation of the inhibition efficiency of a green inhibitor on corrosion of Cu-Ni alloys in the marine application. Key Eng. Mater.786, 174–119 (2018).
LinkOut - more resources
Full Text Sources
Miscellaneous

