Application of machine learning algorithms to identify serological predictors of COVID-19 severity and outcomes
- PMID: 39592832
- PMCID: PMC11599591
- DOI: 10.1038/s43856-024-00658-w
Application of machine learning algorithms to identify serological predictors of COVID-19 severity and outcomes
Abstract
Background: Critically ill hospitalized patients with COVID-19 have greater antibody titers than those with mild to moderate illness, but their association with recovery or death from COVID-19 has not been characterized.
Methods: In a cohort study of 178 COVID-19 patients, 73 non-hospitalized and 105 hospitalized patients, mucosal swabs and plasma samples were collected at hospital enrollment and up to 3 months post-enrollment (MPE) to measure virus RNA, cytokines/chemokines, binding antibodies, ACE2 binding inhibition, and Fc effector antibody responses against SARS-CoV-2. The association of demographic variables and more than 20 serological antibody measures with intubation or death due to COVID-19 was determined using machine learning algorithms.
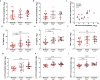
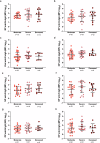
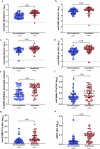
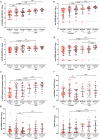
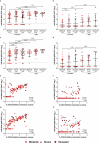
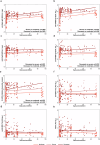
Results: Predictive models reveal that IgG binding and ACE2 binding inhibition responses at 1 MPE are positively and anti-Spike antibody-mediated complement activation at enrollment is negatively associated with an increased probability of intubation or death from COVID-19 within 3 MPE.
Conclusions: At enrollment, serological antibody measures are more predictive than demographic variables of subsequent intubation or death among hospitalized COVID-19 patients.
Plain language summary
Part of the adaptive immune response to viruses, such as SARS-CoV-2, is production of antibodies that are specific to the virus. Hospitalized patients with severe COVID-19 produce more antibodies against SARS-CoV-2 than patients with mild to moderate disease. We studied antibody responses in people with COVID-19 until either recovery or death from the disease. Among hospitalized patients, we analyzed factors, including demographic characteristics, comorbidities, and antibody features that could be used to predict the requirement of intubation or the occurrence of death from COVID-19. We found that antibody measurements taken when people were admitted to the hospital were better at predicting adverse COVID-19 outcomes than either demographic characteristics or comorbidities. These predictive measurements could be useful indicators of disease severity during future pandemics.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Update of
-
Application of machine learning models to identify serological predictors of COVID-19 severity and outcomes.Res Sq [Preprint]. 2023 Nov 13:rs.3.rs-3463155. doi: 10.21203/rs.3.rs-3463155/v1. Res Sq. 2023. Update in: Commun Med (Lond). 2024 Nov 26;4(1):249. doi: 10.1038/s43856-024-00658-w. PMID: 38014049 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

