MDMA pharmacokinetics: A population and physiologically based pharmacokinetics model-informed analysis
- PMID: 39592887
- PMCID: PMC11812931
- DOI: 10.1002/psp4.13282
MDMA pharmacokinetics: A population and physiologically based pharmacokinetics model-informed analysis
Abstract
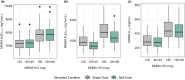
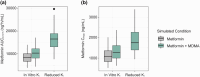
Midomafetamine (3,4-methylenedioxymethamphetamine [MDMA]) is under the U.S. Food and Drug Administration review for treatment of post-traumatic stress disorder in adults. MDMA is metabolized by CYP2D6 and is a strong inhibitor of CYP2D6, as well as a weak inhibitor of renal transporters MATE1, OCT1, and OCT2. A pharmacokinetic phase I study was conducted to evaluate the effects of food on MDMA pharmacokinetics. The results of this study, previously published pharmacokinetic data, and in vitro data were combined to develop and verify MDMA population pharmacokinetic and physiologically based pharmacokinetic models. The food effect study demonstrated that a high-fat/high-calorie meal did not alter MDMA plasma concentrations, but delayed Tmax. The population pharmacokinetic model did not identify any clinically meaningful covariates, including age, weight, sex, race, and fed status. The physiologically based pharmacokinetic model simulated pharmacokinetics for the proposed 120 and 180 mg MDMA HCl clinical doses under single- and split-dose (2 h apart) conditions, indicating minor differences in overall exposure, but lower AUC within the first 4 h and delayed Tmax when administered as a split dose compared to a single dose. The physiologically based pharmacokinetic model also investigated the drug-drug interaction magnitude by varying the fraction metabolized by a representative CYP2D6 substrate (atomoxetine) and evaluated inhibition of renal transporters. The simulations confirm MDMA is a potent CYP2D6 inhibitor, but likely has no meaningful impact on the pharmacokinetics of drugs sensitive to renal transport. This model-informed drug development approach was employed to inform drug-drug interaction potential and predict pharmacokinetics of clinically relevant dosing regimens of MDMA.
© 2024 Lykos Therapeutics. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
MAH, WBS, AV, CL, and RB received payment from Lykos Therapeutics for support with the research. AV is a contractor of Lykos Therapeutics. NBM is an employee of and has stock/stock options in Lykos Therapeutics. BY‐K is an employee of and has stock/stock options in Lykos Therapeutics where she is the Chief Scientific Officer, was previously an employee of MAPS, and has received support from Lykos Therapeutics and MAPS for attending meetings/travel. All other authors declared no competing interests in this work. No authors were compensated for authorship activities associated with this article.
Figures


Similar articles
-
Sex differences in 3,4-methylenedioxymethamphetamine (MDMA; ecstasy)-induced cytochrome P450 2D6 inhibition in humans.Clin Pharmacokinet. 2011 May;50(5):319-29. doi: 10.2165/11584550-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21456632 Clinical Trial.
-
Contribution of cytochrome P450 2D6 to 3,4-methylenedioxymethamphetamine disposition in humans: use of paroxetine as a metabolic inhibitor probe.Clin Pharmacokinet. 2005;44(6):649-60. doi: 10.2165/00003088-200544060-00006. Clin Pharmacokinet. 2005. PMID: 15910012 Clinical Trial.
-
Evaluation of the Ecstasy influence on tramadol and its main metabolite plasma concentration in rats.Drug Metab Pers Ther. 2017 Sep 26;32(3):137-145. doi: 10.1515/dmpt-2017-0018. Drug Metab Pers Ther. 2017. PMID: 28917081
-
MDMA interactions with pharmaceuticals and drugs of abuse.Expert Opin Drug Metab Toxicol. 2020 May;16(5):357-369. doi: 10.1080/17425255.2020.1749262. Epub 2020 Apr 12. Expert Opin Drug Metab Toxicol. 2020. PMID: 32228243 Review.
-
Ecstasy: pharmacodynamic and pharmacokinetic interactions.Psychosomatics. 2004 Jan-Feb;45(1):84-7. doi: 10.1176/appi.psy.45.1.84. Psychosomatics. 2004. PMID: 14709765 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

