Clinical application of capsule sponge testing in symptomatic reflux disease: a national prospective cohort study
- PMID: 39592951
- PMCID: PMC11590222
- DOI: 10.1186/s12876-024-03503-5
Clinical application of capsule sponge testing in symptomatic reflux disease: a national prospective cohort study
Abstract
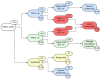
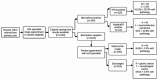
Background: Capsule sponge testing, using an oesophageal cell collection device with biomarkers, was implemented nationally across Scotland in 2020 for symptomatic reflux patients referred to secondary care for non-urgent endoscopy. The aim was to use capsule sponge testing as a triage tool to reduce pressures on the endoscopy service during COVID-19, focus endoscopy resources on those most likely to have pathology and streamline the patient pathway. This prospective cohort study presents the first real-world results and evaluates the clinical application of capsule sponge testing in symptomatic reflux disease based on endoscopic biopsy results.
Methods: Over a 32-month period, all patients undergoing capsule sponge testing for investigation of reflux symptoms across 11 Scottish health boards were identified from prospectively maintained databases. Individual patient records were interrogated to collect baseline demographics, capsule sponge test result (TFF3/atypia/p53) and ongoing clinical management. Further analysis was performed on patients who subsequently underwent upper gastrointestinal (UGI) endoscopy.
Results: 1385 tests were performed for reflux symptoms in 1305 patients. The median follow-up time was 20 months (IQR 12-27). 1103/1385 tests (79.6%) were negative for biomarkers. 913/1305 (70.0%) patients were discharged with no additional investigation required. 355/1305 patients (27.2%) underwent UGI endoscopy due to a positive or insufficient result or ongoing symptoms. With insufficient tests excluded, 52/314 patients (16.6%) had intestinal metaplasia (IM) on endoscopic biopsies, which strongly correlated with positive biomarkers (88.5% vs. 11.5%; p < 0.001), including 1 case of dysplasia. 10/1103 sponge negative patients (0.9%) had biopsies demonstrating IM (n = 6) or malignancy (n = 4): 1 patient was diagnosed with oesophageal adenocarcinoma 27 months later and 3 patients had gastric malignancy, which relies on symptom assessment to direct to endoscopy since the capsule sponge is an oesophageal test.
Conclusions: Capsule sponge testing is effectively at identifying the 30% of symptomatic reflux patients requiring further investigation with UGI endoscopy and aiding the diagnosis of Barrett's oesophagus in clinical practice. Judicious follow-up of the discharged group will be critical to validate the ongoing use of capsule sponge testing long-term.
Keywords: Barrett’s oesophagus; Capsule sponge testing; Gastro-oesophageal reflux disease; Reflux.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was obtained via local information governance teams in each health board, in addition to Public Benefit and Privacy (PBPP) approval on a national level from the Caldicott guardian (NHS Scotland Public Benefit and Privacy Panel for Health and Social Care; NHS Scotland). This was approved with the need for individual patient consent waived due to the retrospective nature of data collection and for the purposes of service development as per NHS Scotland PBPP for Health and Social Care. All patients signed a National Health Service (NHS) consent form prior to the procedure. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340(11):825–31. - PubMed
-
- El-Serag HB, Naik AD, Duan Z, Shakhatreh M, Helm A, Pathak A, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut. 2016;65(8):1252–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous