Carrageenan and insulin resistance in humans: a randomised double-blind cross-over trial
- PMID: 39593091
- PMCID: PMC11590543
- DOI: 10.1186/s12916-024-03771-8
Carrageenan and insulin resistance in humans: a randomised double-blind cross-over trial
Abstract
Background: The potential impact of specific food additives, common in Western diets, on the risk of developing type 2 diabetes is not well understood. This study focuses on carrageenan, a widely used food additive known to induce insulin resistance and gut inflammation in animal models, and its effects on human health.
Methods: In a randomised, double-blind, placebo-controlled, cross-over trial conducted at a university hospital metabolic study centre, 20 males (age 27.4 ± 4.3 years, BMI 24.5 ± 2.5 kg/m2) participated. The intervention involved oral intake of carrageenan (250 mg) or placebo in the morning and in the evening and each intervention lasted 2 weeks. The primary outcome measured was insulin sensitivity (using oral glucose tolerance test [OGTT] and hyperinsulinaemic-euglycaemic clamp). Additional end-points included whole body and hepatic insulin sensitivity, MRI-measured brain inflammation and insulin resistance, intestinal permeability (via lactulose-mannitol test and plasma zonulin levels), and gut microbiome composition. Immune-cell activation and pro-inflammatory cytokine release from peripheral blood mononuclear cells were measured.
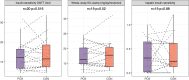
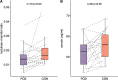
Results: Overall insulin sensitivity did not show significant differences between the treatments. However, interactions between BMI and treatment were observed (OGTT-based insulin sensitivity index: p=0.04, fasting insulin resistance: p=0.01, hepatic insulin sensitivity index: p=0.04). In overweight participants, carrageenan exposure resulted in lower whole body and hepatic insulin sensitivity, a trend towards increased brain inflammation, and elevated C-reactive protein (CRP) and IL-6 levels compared to placebo. Additionally, carrageenan was associated with increased intestinal permeability. In vitro natural killer (NK-)cell activation and increased pro-inflammatory cytokine release were found after carrageenan exposure in the participant's peripheral blood mononuclear cells.
Conclusions: These findings suggest that carrageenan, a common food additive, may contribute to insulin resistance and subclinical inflammation in overweight individuals through pro-inflammatory mechanisms in the gut. Further investigation into the long-term health impacts of carrageenan and other food additives is warranted.
Trial registration: NCT02629705.
Keywords: Carrageenan; Emulsifiers; Gut microbiome; Insulin sensitivity; Intestinal permeability; Type 2 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethical Committee of the Medical Faculty of the University of Tübingen, Germany (project number 359/2012BO1). All participants provided written informed consent. Consent for publication: All authors have reviewed and approved the manuscript for submission. Competing interests: RW reports lecture fees from NovoNordisk, Eli Lilly, Boehringer-Ingelheim and Sanofi, and travel grants from Eli Lilly, Sanofi and NovoNordisk. He served on the advisory board of Akcea Therapeutics, Daiichi Sankyo, Sanofi and NovoNordisk. Outside of the current work, MH reports research grants from Boehringer Ingelheim and Sanofi to the University Hospital of Tübingen, participation in advisory board for Boehringer Ingelheim and Sanofi and lecture fees from Amryt, Bayer, Sanofi, Eli Lilly, Novo Nordisk and Boehringer Ingelheim.
Figures


References
-
- Shah ZC, Huffman FG. Current availability and consumption of carrageenan-containing foods. Ecol Food Nutr. 2003;42(6):357–71.
-
- Weiner ML. Food additive carrageenan: part II: a critical review of carrageenan in vivo safety studies. Crit Rev Toxicol. 2014;44(3):244–69. - PubMed
-
- Tobacman JK, Wallace RB, Zimmerman MB. Consumption of carrageenan and other water-soluble polymers used as food additives and incidence of mammary carcinoma. Med Hypotheses. 2001;56(5):589–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

