Current insights in ultra-rare adenylosuccinate synthetase 1 myopathy - meeting report on the First Clinical and Scientific Conference. 3 June 2024, National Centre for Advancing Translational Science, Rockville, Maryland, the United States of America
- PMID: 39593137
- PMCID: PMC11590305
- DOI: 10.1186/s13023-024-03429-x
Current insights in ultra-rare adenylosuccinate synthetase 1 myopathy - meeting report on the First Clinical and Scientific Conference. 3 June 2024, National Centre for Advancing Translational Science, Rockville, Maryland, the United States of America
Abstract
The inaugural Clinical and Scientific Conference on Adenylosuccinate Synthetase 1 (ADSS1) myopathy was held on June 3, 2024, at the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) in Rockville, Maryland, USA.
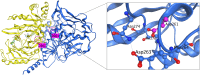
ADSS1 myopathy is an ultra-rare, inherited neuromuscular disease.
Features of geographical patient clusters in South Korea, Japan, India and the United States of America were characterised and discussed.
Pre-clinical animal and cell-based models were discussed, providing unique insight into disease pathogenesis.
The biochemical pathogenesis was discussed, and potential therapeutic targets identified.
Potential clinical and pre-clinical biomarkers were discussed.
An ADSS1 myopathy consortium was established and a roadmap for therapeutic development created.
Keywords: ADSS1 myopathy; Adenylosuccinate synthetase 1 myopathy; Biomarkers; Cardiac muscle; Clinical presentation; Consortium; Guidelines; Inborn error of metabolism; Pre-clinical models; Purine disorder; Skeletal muscle; Therapeutics; Ultra-rare neuromuscular disease.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Human and animal studies were approved by the relevant institutional ethics boards and informed consent was given by all human participants. Consent for publication: Not applicable. Competing interests: ER has received consulting income from Santhera Pharmaceutical and Epirium Bio. PBS has received personal compensation from Novartis Gene Therapies Inc., Alexion argenyx, Biogen, Catalyst, Grifols, CSL Behring, Genentech, Pfizer, PTC Therapeutics, and Sarepta Therapeutics; research funding from Astellas Gene Therapies, Biogen, Catalyst, Pfizer, PTC, Sanofi-Genzyme, Sarepta, Solid Biosciences, and Zogenix. DM founded Kinea Bio Inc., is on the scientific advisory board of Curi Bio Inc., Vita Therapeutics Inc., and SoundEats Inc., and receives consulting income from Astellas Gene Therapies. AHB receives consulting income from Kate Therapeutics, Roche Pharmaceuticals, GLG Inc., and Guidepoint Global, and has equity in Kate Therapeutics and Kinea Bio. All other authors declare no competing interests.
Figures

References
-
- CureADDSL1. www.cure-adssl1.org.
LinkOut - more resources
Full Text Sources

