Modulation of gut microbiota in targeted cancer therapy: insights on the EGFR/VEGF/KRAS pathways
- PMID: 39593276
- PMCID: PMC11745089
- DOI: 10.20892/j.issn.2095-3941.2024.0320
Modulation of gut microbiota in targeted cancer therapy: insights on the EGFR/VEGF/KRAS pathways
Abstract
The rise in the incidence of cancer globally has led to a heightened interest in targeted therapies as a form of anticancer treatment. Key oncogenic targets, including epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and kirsten rat sarcoma viral oncogene homologue (KRAS), have emerged as focal points in the development of targeted agents. Research has investigated the impact of gut microbiota on the efficacy of various anticancer therapies, such as immunotherapy, chemotherapy, and radiotherapy. However, a notable gap exists in the literature regarding the relationship between gut microbiota and targeted agents. This review emphasizes how specific gut microbiota and gut microbiota metabolites, including butyrate, propionate, and ursodeoxycholic acid, interact with oncogenic pathways to modulate anti-tumor effects. Conversely, deoxycholic acid, lipopolysaccharide, and trimethylamine n-oxide may exert pro-tumor effects. Furthermore, modulation of the gut microbiota influences glucose and lipid metabolism, thereby enhancing the response to anti-KRAS agents and addressing diarrhea induced by tyrosine kinase inhibitors. By elucidating the connection between gut microbiota and the EGFR/VEGF/KRAS pathways, this review provides valuable insights for advancing targeted cancer therapy and optimizing treatment outcomes in clinical settings.
Keywords: EGFR; Gut microbiota; KRAS; VEGF; metabolites; targeted therapy; tumorigenesis pathway.
Copyright © 2024 The Authors.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures
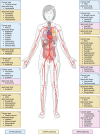
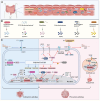
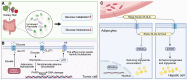
References
-
- Ke X, Shen L. Molecular targeted therapy of cancer: the progress and future prospect. Front Lab Med. 2017;1:69–75.
-
- Wei J, Zheng Z, Hou X, Jia F, Yuan Y, Yuan F, et al. Echinacoside inhibits colorectal cancer metastasis via modulating the gut microbiota and suppressing the PI3K/AKT signaling pathway. J Ethnopharmacol. 2024;318:116866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81920108027/Major International (Regional) Joint Research Program of the National Natural Science Foundation of China
- Y121/National Outstanding Youth Reserve Talent Training Project, Research Capacity Enhancement Project of Chongqing University Cancer Hospital
- Chongqing Young and Middle-Aged Medical Excellence Team
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
