Critical Design Considerations for Longer-Term Wear and Comfort of On-Body Medical Devices
- PMID: 39593718
- PMCID: PMC11591458
- DOI: 10.3390/bioengineering11111058
Critical Design Considerations for Longer-Term Wear and Comfort of On-Body Medical Devices
Abstract
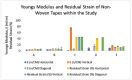
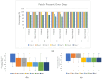
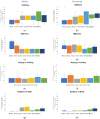
The commercialization of a growing number of wearable devices has been enabled within recent years due to the availability of miniaturized sensor modalities, the development of new materials, and the scalability of flexible electronics. With the increase in resource shortages within healthcare, there is a demand to translate wearable devices from the commercial consumer stand-point to the medical field. Clinical-grade signal quality, wearability, and comfort all need to be tailored to a wearable design. Wear and comfort for user compliance and durability for longer-term use are commonly overlooked. In this study, the relationship of on-body location and material layer composition is investigated. Five non-woven medical tapes noted for longer wear time are tested over a 7-day timeframe. The impact of material properties, such as elasticity, isotropy, and hysteresis, as well as the moisture vapor transmission rate (MVTR) and adhesive thickness, are evaluated in relation to skin properties on the lower torso of 30, high-activity-level volunteers. User perception was quantified via Likert-scale questionnaires and images were obtained for the material-skin interaction. The results indicate that critical characteristics, such as MVTR and elasticity, noted for positive skin interaction in commercial products, may not translate to improved user perception and durability over time. Future work will assess new design options to manipulate material properties for improved wear and comfort.
Keywords: MVTR; higher activity; non-woven tapes; skin-based wearable devices; user perception; wear and comfort.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- A Fruytier L., Janssen D.M., Jurado I.C., van de Sande D.A., Lorato I., Stuart S., Panditha P., de Kok M., MC Kemps H. The Utility of a Novel Electrocardiogram Patch Using Dry Electrodes Technology for Arrhythmia Detection During Exercise and Prolonged Monitoring: Proof-of-Concept Study. JMIR Form. Res. 2023;7:e49346. doi: 10.2196/49346. - DOI - PMC - PubMed
-
- Kulkarni M.B., Rajagopal S., Prieto-Simón B., Pogue B.W., Kulkarni M.B., Rajagopal S., Prieto-Simón B., Pogue B.W., Kulkarni M.B., Rajagopal S., et al. Recent advances in smart wearable sensors for continuous human health monitoring. Talanta. 2024;272:125817. doi: 10.1016/j.talanta.2024.125817. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

