Mitochondrial Reactive Oxygen Species Dysregulation in Heart Failure with Preserved Ejection Fraction: A Fraction of the Whole
- PMID: 39594472
- PMCID: PMC11591317
- DOI: 10.3390/antiox13111330
Mitochondrial Reactive Oxygen Species Dysregulation in Heart Failure with Preserved Ejection Fraction: A Fraction of the Whole
Abstract
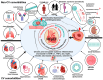
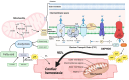
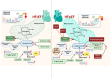
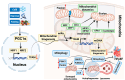
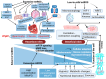
Heart failure with preserved ejection fraction (HFpEF) is a multifarious syndrome, accounting for over half of heart failure (HF) patients receiving clinical treatment. The prevalence of HFpEF is rapidly increasing in the coming decades as the global population ages. It is becoming clearer that HFpEF has a lot of different causes, which makes it challenging to find effective treatments. Currently, there are no proven treatments for people with deteriorating HF or HFpEF. Although the pathophysiologic foundations of HFpEF are complex, excessive reactive oxygen species (ROS) generation and increased oxidative stress caused by mitochondrial dysfunction seem to play a critical role in the pathogenesis of HFpEF. Emerging evidence from animal models and human myocardial tissues from failed hearts shows that mitochondrial aberrations cause a marked increase in mitochondrial ROS (mtROS) production and oxidative stress. Furthermore, studies have reported that common HF medications like beta blockers, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, and mineralocorticoid receptor antagonists indirectly reduce the production of mtROS. Despite the harmful effects of ROS on cardiac remodeling, maintaining mitochondrial homeostasis and cardiac functions requires small amounts of ROS. In this review, we will provide an overview and discussion of the recent findings on mtROS production, its threshold for imbalance, and the subsequent dysfunction that leads to related cardiac and systemic phenotypes in the context of HFpEF. We will also focus on newly discovered cellular and molecular mechanisms underlying ROS dysregulation, current therapeutic options, and future perspectives for treating HFpEF by targeting mtROS and the associated signal molecules.
Keywords: cardiac diastolic dysfunction; cardiovascular disease; heart failure; heart failure with preserved ejection fraction (HFpEF); mitochondrial; mitochondrial dysfunction; oxidative stress; reactive oxygen species; redox signal.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.J Am Coll Cardiol. 2013 Jul 23;62(4):263-71. doi: 10.1016/j.jacc.2013.02.092. Epub 2013 May 15. J Am Coll Cardiol. 2013. PMID: 23684677 Review.
-
Management of Heart Failure With Preserved Ejection Fraction in Elderly Patients: Effectiveness and Safety.Cureus. 2023 Feb 15;15(2):e35030. doi: 10.7759/cureus.35030. eCollection 2023 Feb. Cureus. 2023. PMID: 36938226 Free PMC article. Review.
-
Cardiac diastolic and autonomic dysfunction are aggravated by central chemoreflex activation in heart failure with preserved ejection fraction rats.J Physiol. 2017 Apr 15;595(8):2479-2495. doi: 10.1113/JP273558. Epub 2017 Mar 19. J Physiol. 2017. PMID: 28181258 Free PMC article.
-
NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction.Antioxidants (Basel). 2022 Sep 16;11(9):1822. doi: 10.3390/antiox11091822. Antioxidants (Basel). 2022. PMID: 36139898 Free PMC article. Review.
-
Baseline Characteristics of Patients With Heart Failure and Preserved Ejection Fraction in the PARAGON-HF Trial.Circ Heart Fail. 2018 Jul;11(7):e004962. doi: 10.1161/CIRCHEARTFAILURE.118.004962. Circ Heart Fail. 2018. PMID: 29980595 Clinical Trial.
Cited by
-
Novel Drug Targets in Diastolic Heart Disease.Int J Mol Sci. 2025 Aug 20;26(16):8055. doi: 10.3390/ijms26168055. Int J Mol Sci. 2025. PMID: 40869375 Free PMC article. Review.
-
Targeting Mitochondrial Dysfunction in Cerebral Ischemia: Advances in Pharmacological Interventions.Antioxidants (Basel). 2025 Jan 18;14(1):108. doi: 10.3390/antiox14010108. Antioxidants (Basel). 2025. PMID: 39857442 Free PMC article. Review.
-
Emerging Role of Hypoxia-Inducible Factors (HIFs) in Modulating Autophagy: Perspectives on Cancer Therapy.Int J Mol Sci. 2025 Feb 19;26(4):1752. doi: 10.3390/ijms26041752. Int J Mol Sci. 2025. PMID: 40004215 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

