Role of Paraoxonase 2 in Airway Epithelial Response to Oxidant Stress
- PMID: 39594475
- PMCID: PMC11591210
- DOI: 10.3390/antiox13111333
Role of Paraoxonase 2 in Airway Epithelial Response to Oxidant Stress
Abstract
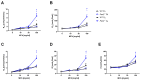
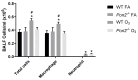
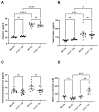
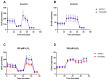
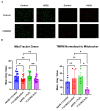
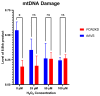
Asthma is a widespread chronic lung disease characterized by airway inflammation and hyperresponsiveness. This airway inflammation is classified by either the presence (T2-high) or absence (T2-low) of high levels of eosinophils. Because most therapies for asthma target eosinophils and related pathways, treatment options for T2-low disease are limited. New pathophysiologic targets are needed. Oxidant stress is a common feature of T2-low disease. Airway epithelial expression of the antioxidant enzyme Paraoxonase 2 (PON2) is decreased in a well-recognized population of people with T2-low asthma and people with obesity and asthma. As a potential mechanism of increased oxidant stress, we measured the role of PON2 in lung oxidant responses using an environmentally relevant in vivo murine oxidant exposure (i.e., ozone) and in vitro studies with an immortalized human airway epithelial cell line BEAS-2B. Pon2-deficient (Pon2-/-) mice developed increased airway hyper-responsiveness compared to wild-type controls. Despite reduced alveolar macrophage influx, Pon2-/- mice exhibited increased nitrite production. In human airway epithelial cells incubated with hydrogen peroxide, PON2 knockdown (PON2KD) decreased mitochondrial function and inner mitochondrial membrane potential. These findings suggest that PON2 functions in defending against airway epithelial oxidant stress. Further studies are needed to elucidate the mechanisms linking PON2, oxidant stress, and asthma pathogenesis.
Keywords: asthma; mitochondria; oxidant stress; ozone; paraoxonase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

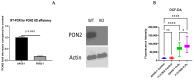
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

