Role of Plant Phytochemicals: Resveratrol, Curcumin, Luteolin and Quercetin in Demyelination, Neurodegeneration, and Epilepsy
- PMID: 39594506
- PMCID: PMC11591432
- DOI: 10.3390/antiox13111364
Role of Plant Phytochemicals: Resveratrol, Curcumin, Luteolin and Quercetin in Demyelination, Neurodegeneration, and Epilepsy
Abstract
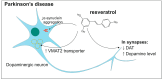
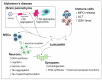
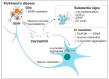
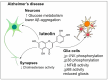
Polyphenols are an important group of biologically active compounds present in almost all food sources of plant origin and are primarily known for their anti-inflammatory and antioxidative capabilities. Numerous studies have indicated their broad spectrum of pharmacological properties and correlations between their increased supply in the human diet and lower prevalence of various disorders. The positive effects of polyphenols application are mostly discussed in terms of cardiovascular system well-being. However, in recent years, they have also increasingly mentioned as prophylactic and therapeutic factors in the context of neurological diseases, being able to suppress the progression of such disorders and soothe accompanying symptoms. Among over 8000 various compounds, that have been identified, the most widely examined comprise resveratrol, curcumin, luteolin and quercetin. This review focuses on in vitro assessments, animal models and clinical trials, reflecting the most actual state of knowledge, of mentioned polyphenols' medicinal capabilities in epilepsy, demyelinating and neurodegenerative diseases of the central nervous system.
Keywords: curcumin; demyelinating diseases; epilepsy; luteolin; neurodegenerative diseases; polyphenols; quercetin; resveratrol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
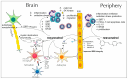
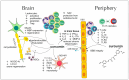
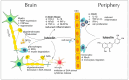
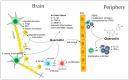

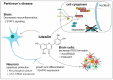

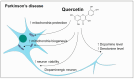
References
Publication types
LinkOut - more resources
Full Text Sources

