Potential Vitamin E Signaling Mediators in Skeletal Muscle
- PMID: 39594525
- PMCID: PMC11591548
- DOI: 10.3390/antiox13111383
Potential Vitamin E Signaling Mediators in Skeletal Muscle
Abstract
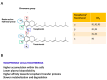
Vitamin E (Vit E) deficiency studies underline the relevance of this vitamin in skeletal muscle (SkM) homeostasis. The knowledge of the effectors and modulators of Vit E action in SkM cells is limited, especially in aging and chronic diseases characterized by a decline in musculoskeletal health. Vit E comprises eight fat-soluble compounds grouped into tocopherols and tocotrienols, which share the basic chemical structure but show different biological properties and potentials to prevent diseases. Vit E has antioxidant and non-antioxidant activities and both favorable and adverse effects depending on the specific conditions and tissues. In this review, we focus on the actual knowledge of Vit E forms in SkM functions and new potential signaling effectors (i.e., bioactive sphingolipids and myokines). The possible advantages of Vit E supplementation in counteracting SkM dysfunctions in sarcopenia and under microgravity will also be discussed.
Keywords: antioxidant action; microgravity; myokines; sarcopenia; skeletal muscle; sphingolipids; vitamin E.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of this manuscript; or in the decision to publish the results.
Figures


References
-
- Szewczyk K., Niki E., Abe K. Vitamin E: Chemistry and Nutritional Benefits. Int. J. Mol. Sci. 2021;9:6222. doi: 10.3390/ijms22126222. - DOI
-
- Morrissey P.A., Kiely M. Vitamin E: Physiology and health effects. In: Caballer B., Allen L., Prentice A., editors. Encyclopedia of Human Nutrition. 2nd ed. Elsevier; Amsterdam, The Netherlands: Academic Press; Cambridge, MA, USA: 2005. pp. 389–398.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

