Nitroxyl Hybrids with Curcumin and Stilbene Scaffolds Display Potent Antioxidant Activity, Remodel the Amyloid Beta Oligomer, and Reverse Amyloid Beta-Induced Cytotoxicity
- PMID: 39594552
- PMCID: PMC11591036
- DOI: 10.3390/antiox13111411
Nitroxyl Hybrids with Curcumin and Stilbene Scaffolds Display Potent Antioxidant Activity, Remodel the Amyloid Beta Oligomer, and Reverse Amyloid Beta-Induced Cytotoxicity
Abstract
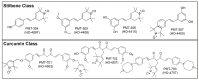
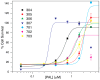
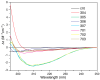
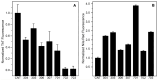
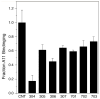
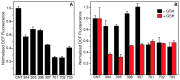
The disorder and heterogeneity of low-molecular-weight amyloid-beta oligomers (AβOs) underlie their participation in multiple modes of cellular dysfunction associated with the etiology of Alzheimer's disease (AD). The lack of specified conformational states in these species complicates efforts to select or design small molecules to targeting discrete pathogenic states. Furthermore, targeting AβOs alone may be therapeutically insufficient, as AD progresses as a multifactorial, self-amplifying cascade. To address these challenges, we have screened the activity of seven new candidates that serve as Paramagnetic Amyloid Ligand (PAL) candidates. PALs are bifunctional small molecules that both remodel the AβO structure and localize a potent antioxidant that mimics the activity of SOD within live cells. The candidates are built from either a stilbene or curcumin scaffold with nitroxyl moiety to serve as catalytic antioxidants. Measurements of PAL AβO binding and remolding along with assessments of bioactivity allow for the extraction of useful SAR information from screening data. One candidate (HO-4450; PMT-307), with a six-membered nitroxyl ring attached to a stilbene ring, displays the highest potency in protecting against cell-derived Aβ. A preliminary low-dose evaluation in AD model mice provides evidence of modest treatment effects by HO-4450. The results for the curcumin PALs demonstrate that the retention of the native curcumin phenolic groups is advantageous to the design of the hybrid PAL candidates. Finally, the PAL remodeling of AβO secondary structures shows a reasonable correlation between a candidate's bioactivity and its ability to reduce the fraction of antiparallel β-strand.
Keywords: Alzheimer’s disease; Aβ oligomer; EPR; amyloid beta peptide; antioxidant; bifunctional drug; electron paramagnetic resonance spectroscopy; nitroxide; oxidative stress; protein misfolding.
Conflict of interest statement
Authors J.S. and J.C.V. were employed by the company Paramag, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Sperling R.A., Donohue M.C., Raman R., Sun C.K., Yaari R., Holdridge K., Siemers E., Johnson K.A., Aisen P.S., A4 Study Team Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals. JAMA Neurol. 2020;77:735–745. doi: 10.1001/jamaneurol.2020.0387. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

