The Adhesion GPCR ADGRL2/LPHN2 Can Protect Against Cellular and Organismal Dysfunction
- PMID: 39594576
- PMCID: PMC11592504
- DOI: 10.3390/cells13221826
The Adhesion GPCR ADGRL2/LPHN2 Can Protect Against Cellular and Organismal Dysfunction
Abstract
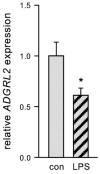
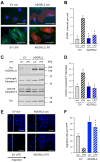
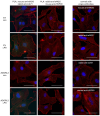
The most common trigger of sepsis and septic shock is bacterial lipopolysaccharide (LPS). Endothelial cells are among the first to encounter LPS directly. Generally, their function is closely linked to active endothelial NO Synthase (eNOS), which is significantly reduced under septic conditions. LPS treatment of endothelial cells leads to their activation and apoptosis, resulting in loss of integrity and vascular leakage, a hallmark of septic shock. Hence, therapies that prevent endothelial leakage or restore the endothelial barrier would be invaluable for patients. Adhesion GPCRs (aGPCRs) have been largely overlooked in this context, although particularly one of them, ADGRL2/LPHN2, has been implicated in endothelial barrier function. Our study shows that overexpression of ADGRL2 protects endothelial cells from LPS-induced activation, apoptosis, and impaired migration. Mechanistically, ADGRL2 preserves eNOS activity by shifting its binding from Caveolin-1 to Heat Shock Protein 90. Furthermore, ADGRL2 enhances antioxidative responses by increasing NRF2 activity. Notably, we found that this function may be evolutionarily conserved. In the absence of lat-2, a homolog of ADGRL2 in Caenorhabditis elegans, worms show higher ROS levels and altered stress response gene expression. Additionally, lat-2 mutants have a significantly reduced lifespan, altogether indicating a protective role of ADGRL2 against oxidative stress across species.
Keywords: ADGRL2/LPHN2; Caenorhabditis elegans; NRF2; eNOS; endothelial cells; lipopolysaccharide; reactive oxygen species; skn-1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Hamann J., Aust G., Arac D., Engel F.B., Formstone C., Fredriksson R., Hall R.A., Harty B.L., Kirchhoff C., Knapp B., et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol. Rev. 2015;67:338–367. doi: 10.1124/pr.114.009647. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

