Short-Term Culture of Human Hyalocytes Retains Their Initial Phenotype and Displays Their Contraction Abilities
- PMID: 39594586
- PMCID: PMC11592754
- DOI: 10.3390/cells13221837
Short-Term Culture of Human Hyalocytes Retains Their Initial Phenotype and Displays Their Contraction Abilities
Abstract
Background: Hyalocytes are the main vitreal cell types with critical functions in health and vitreoretinal diseases. Our aim was to develop cultures of human hyalocytes and verify the retention of their initial cellular features after 3 and 6 days of culturing (3 d and 6 d) by analyzing and comparing a few morphological and functional parameters.
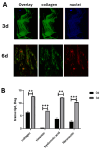
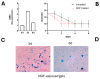
Methods: Vitreous samples (n = 22) were collected and vitreous cells and bead-enriched hyalocytes were developed and compared (3 d vs. 6 d cultures). Vitreous and conditioned media were tested for collagen, vascular endothelial growth factor (VEGF), transforming growth factor β1 (TGFβ1), nerve growth factor (NGF), matrix metalloproteinases (MMPs)/tissue inhibitors of metalloproteinases (TIMPs) and alpha-smooth muscle actin (αSMA) expression (ELISA, array/IP/WB, RT-PCR). Cells were observed at light and fluorescent microscopy (phenotypical properties) and tested for their 3D collagen gel contraction abilities.
Results: An increased expression of collagens, vimentin, fibronectin, and the MMP9/TIMP1 ratio were observed in vitreous tissues. In 3 d cultures, collagens and MMP9 were upregulated while the related tissue-enzymes were deregulated. Vitreous samples also showed high levels of TGFβ1, VEGF, and NGF, and this protein signature was retained at 3 d while decreased at 6 d. The original phenotype (low αSMA) was retained at 3 d from seeding while an increased αSMA expression was observed at 6 d; NGF/trkANGFR was expressed in cultured hyalocytes and partially drives the collagen retraction.
Conclusions: The vitreous print comparison between untouched and cultured hyalocytes allowed us, on one side, to select 3 d cultures and, on the other, to highlight the neuroprotective/contractile NGF in vitro hyalocytes effects. The possibility of scoring reactive hyalocytes would represent an interesting aspect of screening the vitreoretinal interface severity.
Keywords: NGF; hyalocytes; vitreal cells; vitreoretinal diseases; vitreous; αSMA.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Boneva S.K., Wolf J., Hajdú R.I., Prinz G., Salié H., Schlecht A., Killmer S., Laich Y., Faatz H., Lommatzsch A., et al. In-Depth Molecular Characterization of Neovascular Membranes Suggests a Role for Hyalocyte-to-Myofibroblast Transdifferentiation in Proliferative Diabetic Retinopathy. Front. Immunol. 2021;12:757607. doi: 10.3389/fimmu.2021.757607. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

