Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications
- PMID: 39594587
- PMCID: PMC11592877
- DOI: 10.3390/cells13221838
Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications
Abstract
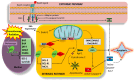
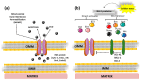
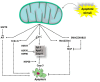
Cell survival and death are intricately governed by apoptosis, a meticulously controlled programmed cell death. Apoptosis is vital in facilitating embryonic development and maintaining tissue homeostasis and immunological functioning. It is a complex interplay of intrinsic and extrinsic signaling pathways that ultimately converges on executing the apoptotic program. The extrinsic pathway is initiated by the binding of death ligands such as TNF-α and Fas to their respective receptors on the cell surface. In contrast, the intrinsic pathway leads to increased permeability of the outer mitochondrial membrane and the release of apoptogenic factors like cytochrome c, which is regulated by the Bcl-2 family of proteins. Once activated, these pathways lead to a cascade of biochemical events, including caspase activation, DNA fragmentation, and the dismantling of cellular components. Dysregulation of apoptosis is implicated in various disorders, such as cancer, autoimmune diseases, neurodegenerative disorders, and cardiovascular diseases. This article focuses on elucidating the molecular mechanisms underlying apoptosis regulation, to develop targeted therapeutic strategies. Modulating apoptotic pathways holds immense potential in cancer treatment, where promoting apoptosis in malignant cells could lead to tumor regression. This article demonstrates the therapeutic potential of targeting apoptosis, providing options for treating cancer and neurological illnesses. The safety and effectiveness of apoptosis-targeting drugs are being assessed in ongoing preclinical and clinical trials (phase I-III), opening the door for more effective therapeutic approaches and better patient outcomes.
Keywords: apoptosis; morphological changes; physiological significance; signaling pathways; therapeutic strategies.
Conflict of interest statement
The authors declare that they have no discernible competing financial interests or personal connections that could be interpreted as influencing the conclusions made in this work.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

