Modelling Functional Thyroid Follicular Structures Using P19 Embryonal Carcinoma Cells
- PMID: 39594593
- PMCID: PMC11593046
- DOI: 10.3390/cells13221844
Modelling Functional Thyroid Follicular Structures Using P19 Embryonal Carcinoma Cells
Abstract
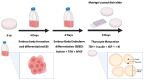
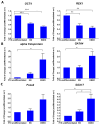
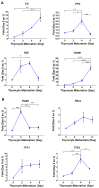
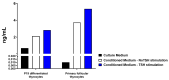
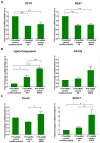
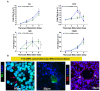
Thyroid gland diseases remain clinical challenges due to the lack of reliable in vitro models to examine molecular pathways of thyrocytes development, maturation, and functional maintenance. This study aimed to develop in vitro thyrocytes model using a stem cell culture, P19 embryonal carcinoma which requires no feeder layer, differentiation into mature and functional thyrocytes that allow molecular and genetic manipulation for studying thyroid diseases. The procedure utilizes Activin A and thyroid stimulating hormone (TSH) to first induce embryoid body endoderm formation enriched in thyrocyte progenitors. Following dissociating embryoid bodies, thyrocyte progenitors are plated in Matrigel as monolayer cultures that allows thyrocyte progenitors mature to functional thyrocytes. These thyrocytes further maturate to form follicle-like structures expressing and accumulating thyroglobulin that can be secreted into the medium upon TSH stimulation. Thyrocyte differentiation-maturation process is monitored by the expression of essential transcriptional factors and thyrocyte-specific functional genes. Further, the applicability of this system is validated by introducing a siRNA control. Following molecular manipulation, the system can still be guided to differentiate into mature and functional thyrocytes. This system spans a time frame of 14 days, suitable for detailed molecular studies to dissect pathways and molecular players in thyrocytes development and functional maintenance.
Keywords: cell differentiation; embryonal carcinoma stem cells; in vitro models; thyrocytes; thyroid diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Tomás G., Tarabichi M., Gacquer D., Hébrant A., Dom G., Dumont J.E., Keutgen X., Fahey T.J., Maenhaut C., Detours V. A general method to derive robust organ-specific gene expression-based differentiation indices: Application to thyroid cancer diagnostic. Oncogene. 2012;31:4490–4498. doi: 10.1038/onc.2011.626. - DOI - PubMed
-
- Najjar F., Nhieu J., Wei C.-W., Milbauer L., Burmeister L., Seelig D., Wei L.-N. Deleting Cellular Retinoic-Acid-Binding Protein-1 (Crabp1) Gene Causes Adult-Onset Primary Hypothyroidism in Mice. Endocrines. 2023;4:138–150. doi: 10.3390/endocrines4010013. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

