Crosstalk Between Oxidative Stress and Epigenetics: Unveiling New Biomarkers in Human Infertility
- PMID: 39594595
- PMCID: PMC11593296
- DOI: 10.3390/cells13221846
Crosstalk Between Oxidative Stress and Epigenetics: Unveiling New Biomarkers in Human Infertility
Abstract
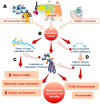
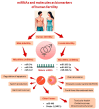
The correlation between epigenetic alterations and the pathophysiology of human infertility is progressively being elucidated with the discovery of an increasing number of target genes that exhibit altered expression patterns linked to reproductive abnormalities. Several genes and molecules are emerging as important for the future management of human infertility. In men, microRNAs (miRNAs) like miR-34c, miR-34b, and miR-122 regulate apoptosis, sperm production, and germ cell survival, while other factors, such as miR-449 and sirtuin 1 (SIRT1), influence testicular health, oxidative stress, and mitochondrial function. In women, miR-100-5p, miR-483-5p, and miR-486-5p are linked to ovarian reserve, PCOS, and conditions like endometriosis. Mechanisms such as DNA methylation, histone modification, chromatin restructuring, and the influence of these non-coding RNA (ncRNA) molecules have been identified as potential perturbators of normal spermatogenesis and oogenesis processes. In fact, alteration of these key regulators of epigenetic processes can lead to reproductive disorders such as defective spermatogenesis, failure of oocyte maturation and embryonic development alteration. One of the primary factors contributing to changes in the key epigenetic regulators appear to be oxidative stress, which arises from environmental exposure to toxic substances or unhealthy lifestyle choices. This evidence-based study, retracing the major epigenetic processes, aims to identify and discuss the main epigenetic biomarkers of male and female fertility associated with an oxidative imbalance, providing future perspectives in the diagnosis and management of infertile couples.
Keywords: DNA modifications; epigenetics; human fertility; reactive oxygen species; reproductive health.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health.Genes (Basel). 2025 Jan 17;16(1):93. doi: 10.3390/genes16010093. Genes (Basel). 2025. PMID: 39858640 Free PMC article. Review.
-
Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis.Andrologia. 2019 Dec;51(11):e13432. doi: 10.1111/and.13432. Epub 2019 Oct 3. Andrologia. 2019. PMID: 31583745 Review.
-
Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility.Hum Reprod Update. 2018 May 1;24(3):267-289. doi: 10.1093/humupd/dmy003. Hum Reprod Update. 2018. PMID: 29447380 Review.
-
Alterations in the sperm histone-retained epigenome are associated with unexplained male factor infertility and poor blastocyst development in donor oocyte IVF cycles.Hum Reprod. 2017 Dec 1;32(12):2443-2455. doi: 10.1093/humrep/dex317. Hum Reprod. 2017. PMID: 29087470
-
Expression and Methylation Pattern of hsa-miR-34 Family in Sperm Samples of Infertile Men.Reprod Sci. 2020 Jan;27(1):301-308. doi: 10.1007/s43032-019-00025-4. Epub 2020 Jan 1. Reprod Sci. 2020. PMID: 32046388
Cited by
-
Comparative analysis of Chlamydia pneumoniae pneumonia (CPP) and Mycoplasma pneumoniae pneumonia in children and risk factors of severe CPP.BMC Infect Dis. 2025 Aug 7;25(1):993. doi: 10.1186/s12879-025-11405-4. BMC Infect Dis. 2025. PMID: 40775274 Free PMC article.
-
Role of Defense/Immunity Proteins in Non-Obstructive Azoospermia: Insights from Gene Expression and Single-Cell RNA Sequencing Analyses.Reprod Sci. 2025 Jul;32(7):2484-2498. doi: 10.1007/s43032-025-01916-5. Epub 2025 Jun 19. Reprod Sci. 2025. PMID: 40537735
-
Association between imprinting disorders and assisted reproductive technologies.Epigenomics. 2025 Apr;17(6):397-410. doi: 10.1080/17501911.2025.2471269. Epub 2025 Mar 3. Epigenomics. 2025. PMID: 40033833 Free PMC article. Review.
-
Redox-Driven Epigenetic Modifications in Sperm: Unraveling Paternal Influences on Embryo Development and Transgenerational Health.Antioxidants (Basel). 2025 May 9;14(5):570. doi: 10.3390/antiox14050570. Antioxidants (Basel). 2025. PMID: 40427452 Free PMC article. Review.
-
Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health.Genes (Basel). 2025 Jan 17;16(1):93. doi: 10.3390/genes16010093. Genes (Basel). 2025. PMID: 39858640 Free PMC article. Review.
References
-
- Sinha A., Singh V., Yadav S. Multi-omics and male infertility: Status, integration and future prospects. Front. Biosci. 2017;9:375 - PubMed
-
- Das S., Guha P., Nath M., Das S., Sen S., Sahu J., Kopanska M., Dutta S., Jamal Q.M.S., Kesari K.K., et al. A Comparative Cross-Platform Analysis to Identify Potential Biomarker Genes for Evaluation of Teratozoospermia and Azoospermia. Genes. 2022;13:1721. doi: 10.3390/genes13101721. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

