Semaglutide Ameliorates Diabetic Neuropathic Pain by Inhibiting Neuroinflammation in the Spinal Cord
- PMID: 39594606
- PMCID: PMC11593193
- DOI: 10.3390/cells13221857
Semaglutide Ameliorates Diabetic Neuropathic Pain by Inhibiting Neuroinflammation in the Spinal Cord
Abstract
Glucagon-like peptide 1 (GLP-1) receptor agonists are frequently used to treat type 2 diabetes and obesity. Despite the development of several drugs for neuropathic pain management, their poor efficacy, tolerance, addiction potential, and side effects limit their usage. Teneligliptin, a DPP-4 inhibitor, has been shown to reduce spinal astrocyte activation and neuropathic pain caused by partial sciatic nerve transection. Additionally, we showed its capacity to improve the analgesic effects of morphine and reduce analgesic tolerance. Recent studies indicate that GLP-1 synthesized in the brain activates GLP-1 receptor signaling pathways, essential for neuroprotection and anti-inflammatory effects. Multiple in vitro and in vivo studies using preclinical models of neurodegenerative disorders have shown the anti-inflammatory properties associated with glucagon-like peptide-1 receptor (GLP-1R) activation. This study aimed to investigate the mechanism of antinociception and the effects of the GLP-1 agonist semaglutide (SEMA) on diabetic neuropathic pain in diabetic rats.
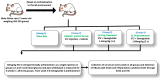
Methods: Male Wistar rats, each weighing between 300 and 350 g, were categorized into four groups: one non-diabetic sham group and three diabetic groups. The diabetic group received a single intraperitoneal injection of streptozotocin (STZ) at a dosage of 60 mg/kg to induce diabetic neuropathy. After 4 weeks of STZ injection, one diabetic group was given saline (vehicle), and the other two were treated with either 1× SEMA (1.44 mg/kg, orally) or 2× SEMA (2.88 mg/kg, orally). Following a 4-week course of oral drug treatment, behavioral, biochemical, and immunohistochemical analyses were carried out. The mechanical allodynia, thermal hyperalgesia, blood glucose, advanced glycation end products (AGEs), plasma HbA1C, and spinal inflammatory markers were evaluated.
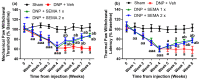
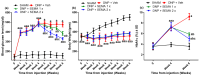
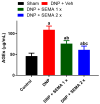
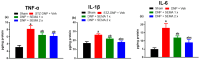
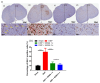
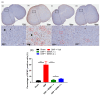
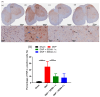
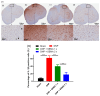
Results: SEMA treatment significantly reduced both allodynia and hyperalgesia in the diabetic group. SEMA therapy had a limited impact on body weight restoration and blood glucose reduction. In diabetic rats, SEMA lowered the amounts of pro-inflammatory cytokines in the spinal cord and dorsal horn. It also lowered the activation of microglia and astrocytes in the dorsal horn. SEMA significantly reduced HbA1c and AGE levels in diabetic rats compared to the sham control group.
Conclusions: These results indicate SEMA's neuroprotective benefits against diabetic neuropathic pain, most likely by reducing inflammation and oxidative stress by inhibiting astrocyte and microglial activity. Our findings suggest that we can repurpose GLP-1 agonists as potent anti-hyperalgesic and anti-inflammatory drugs to treat neuropathic pain without serious side effects.
Keywords: GLP-1RA; SEMA; astrocytes; diabetic neuropathic pain; microglia; pro-inflammatory cytokines.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

