Structural and Functional Insights into Dishevelled-Mediated Wnt Signaling
- PMID: 39594618
- PMCID: PMC11592973
- DOI: 10.3390/cells13221870
Structural and Functional Insights into Dishevelled-Mediated Wnt Signaling
Abstract
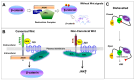
Dishevelled (DVL) proteins precisely control Wnt signaling pathways with many effectors. While substantial research has advanced our understanding of DVL's role in Wnt pathways, key questions regarding its regulatory mechanisms and interactions remain unresolved. Herein, we present the recent advances and perspectives on how DVL regulates signaling. The experimentally determined conserved domain structures of DVL in conjunction with AlphaFold-predicted structures are used to understand the DVL's role in Wnt signaling regulation. We also summarize the role of DVL in various diseases and provide insights into further directions for research on the DVL-mediated signaling mechanisms. These findings underscore the importance of DVL as a pharmaceutical target or biological marker in diseases, offering exciting potential for future biomedical applications.
Keywords: AlphaFold; Dishevelled; Wnt; cancer; mechanism; post-translation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

