Loss of Dnah5 Downregulates Dync1h1 Expression, Causing Cortical Development Disorders and Congenital Hydrocephalus
- PMID: 39594631
- PMCID: PMC11593149
- DOI: 10.3390/cells13221882
Loss of Dnah5 Downregulates Dync1h1 Expression, Causing Cortical Development Disorders and Congenital Hydrocephalus
Abstract
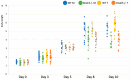
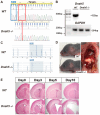
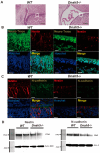
Dnah5 is associated with primary ciliary dyskinesia in humans. Dnah5-knockout (Dnah5-/- mice develop acute hydrocephalus shortly after birth owing to impaired ciliary motility and cerebrospinal fluid (CSF) stagnation. In contrast to chronic adult-onset hydrocephalus observed in other models, this rapid ventricular enlargement indicates additional factors beyond CSF stagnation. Herein, we investigated the contributors to rapid ventricular enlargement in congenital hydrocephalus. Dnah5-/- mice were generated using CRISPR/Cas9. The expression of dynein, N-cadherin, and nestin in the cerebral cortex was assessed using microarrays and immunostaining. Real-time PCR and Western blotting were performed for gene and protein quantification, respectively. All Dnah5-/- mice developed hydrocephalus, confirmed by electron microscopy, indicating the absence of axonemal outer dynein arms. Ventricular enlargement occurred rapidly, with a 25% reduction in the number of mature neurons in the motor cortex. Dync1h1 expression was decreased, while cytoplasmic dynein levels were 56.3% lower. Levels of nestin and N-cadherin in the lateral ventricular walls decreased by 31.7% and 33.3%, respectively. Reduced cytoplasmic dynein disrupts neurogenesis and axonal growth and reduces neuron cortical density. Hydrocephalus in Dnah5-/- mice may result from cortical maldevelopment due to cytoplasmic dynein deficiency, further exacerbating ventricular enlargement due to CSF stagnation caused by impaired motile ciliary function.
Keywords: Dnah5; cytoplasmic dynein; motile cilia; neurogenesis; primary cilia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 22GB1002/Juntendo Research Branding Project, Health, Labor, and Welfare Sciences Research Grants
- 20K09355/Japan Society for the Promotion of Science under Grants-in-Aid for Scientific Research
- 20K09398/Japan Society for the Promotion of Science under Grants-in-Aid for Scientific Research
- 22H03675/Japan Society for the Promotion of Science under Grants-in-Aid for Scientific Research
- 24K10497/Japan Society for the Promotion of Science under Grants-in-Aid for Scientific Research
LinkOut - more resources
Full Text Sources
Medical
Research Materials

