Emerging Role of Extracellular pH in Tumor Microenvironment as a Therapeutic Target for Cancer Immunotherapy
- PMID: 39594672
- PMCID: PMC11592846
- DOI: 10.3390/cells13221924
Emerging Role of Extracellular pH in Tumor Microenvironment as a Therapeutic Target for Cancer Immunotherapy
Abstract
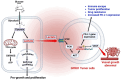
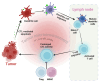
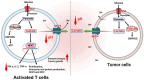
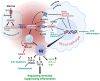
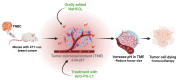
Identifying definitive biomarkers that predict clinical response and resistance to immunotherapy remains a critical challenge. One emerging factor is extracellular acidosis in the tumor microenvironment (TME), which significantly impairs immune cell function and contributes to immunotherapy failure. However, acidic conditions in the TME disrupt the interaction between cancer and immune cells, driving tumor-infiltrating T cells and NK cells into an inactivated, anergic state. Simultaneously, acidosis promotes the recruitment and activation of immunosuppressive cells, such as myeloid-derived suppressor cells and regulatory T cells (Tregs). Notably, tumor acidity enhances exosome release from Tregs, further amplifying immunosuppression. Tumor acidity thus acts as a "protective shield," neutralizing anti-tumor immune responses and transforming immune cells into pro-tumor allies. Therefore, targeting lactate metabolism has emerged as a promising strategy to overcome this barrier, with approaches including buffer agents to neutralize acidic pH and inhibitors to block lactate production or transport, thereby restoring immune cell efficacy in the TME. Recent discoveries have identified genes involved in extracellular pH (pHe) regulation, presenting new therapeutic targets. Moreover, ongoing research aims to elucidate the molecular mechanisms driving extracellular acidification and to develop treatments that modulate pH levels to enhance immunotherapy outcomes. Additionally, future clinical studies are crucial to validate the safety and efficacy of pHe-targeted therapies in cancer patients. Thus, this review explores the regulation of pHe in the TME and its potential role in improving cancer immunotherapy.
Keywords: T cells; acidic TME; extracellular pH; immunotherapy resistance; lactate metabolism; tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lu J., Qi F. Tumor Microenvironment. Tumor Microenviron. Res. 2020;2:42–49. doi: 10.53388/TMER201900012. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

