HDAC6 as a Prognostic Factor and Druggable Target in HER2-Positive Breast Cancer
- PMID: 39594707
- PMCID: PMC11591923
- DOI: 10.3390/cancers16223752
HDAC6 as a Prognostic Factor and Druggable Target in HER2-Positive Breast Cancer
Abstract
Background: Adjuvant trastuzumab is the standard of care for HER2+ breast cancer (BC) patients. However, >50% of patients become resistant. This study aimed at the identification of the molecular factors associated with disease relapse and their further investigation as therapeutically exploitable targets.
Methods: Analyses were conducted on formalin-fixed paraffin-embedded tissues of the primary tumors of relapsed (cases) and not relapsed (controls) HER2+ BC patients treated with adjuvant trastuzumab. The nCounter Human Breast Cancer Panel 360 was used. Logistic regression and partitioning around medoids were employed to identify the genes associated with disease recurrence. Cytotoxicity experiments using trastuzumab-resistant cell lines and a network pharmacology approach were carried out to investigate drug efficacy.
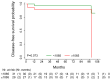
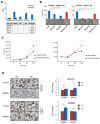
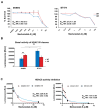
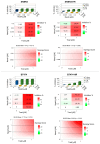
Results: A total of 52 patients (26 relapsed and 26 not relapsed) were analyzed. We found that a higher expression of HDAC6 was significantly associated with an increased risk of recurrence, with an adjusted OR of 3.20 (95% CI 1.38-9.91, p = 0.016). Then, we investigated the cytotoxic activity of the selective HDAC6 inhibitor Nexturastat A (NextA) on HER2+ cell lines, which were both sensitive and trastuzumab-resistant. A sub-cytotoxic concentration of NextA, combined with trastuzumab, showed a synergistic effect on BC cell lines. Finally, using a network pharmacology approach, we identified HSP90AA1 as the putative molecular candidate responsible for the synergism observed in vitro.
Conclusions: Our findings encourage the exploration of the role of HDAC6 as a prognostic factor and the combinatorial use of HDAC6 selective inhibitors combined with trastuzumab in HER2+ BC, in particular for those patients experiencing drug resistance.
Keywords: HDAC6; HER2 positive; adjuvant treatment; breast cancer; resistance; trastuzumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

