Clinical Utility and Diagnostic Accuracy of ROMA, RMI, ADNEX, HE4, and CA125 in the Prediction of Malignancy in Adnexal Masses
- PMID: 39594745
- PMCID: PMC11592863
- DOI: 10.3390/cancers16223790
Clinical Utility and Diagnostic Accuracy of ROMA, RMI, ADNEX, HE4, and CA125 in the Prediction of Malignancy in Adnexal Masses
Abstract
Objective: We aimed to compare the clinical utility and diagnostic accuracy of the ADNEX model, ROMA score, RMI I, and RMI IV, as well as two serum markers (CA125 and HE4) in preoperative discrimination between benign and malignant adnexal masses (AMs).
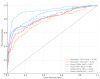
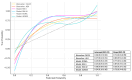
Methods: We conducted a retrospective study extracting all consecutive patients with AMs seen at our Institution between January 2015 and December 2020. Accuracy metrics included sensitivity (SE), specificity (SP), and area under the receiver operating characteristic curve (AUC), and their 95% confidence intervals (CI) were calculated for basic discrimination between AMs. Model performance was evaluated in terms of discrimination ability and clinical utility (net benefit, NB).
Results: A total of 581 women were included; 481 (82.8%) had a benign ovarian tumor and 100 (17.2%) had a malignant tumor. The SE and SP of CA125, HE4, ROMA score, RMI I, RMI IV, and ADNEX model were 0.60 (0.54-0.66) and 0.80 (0.76-0.83); 0.39 (0.30-0.49) and 0.96 (0.94-0.98); 0.59 (0.50-0.68) and 0.92 (0.88-0.95); 0.56 (0.46-0.65) and 0.98 (0.96-0.99); 0.54 (0.44-0.63) and 0.96 (0.94-0.98); 0.82 (0.73-0.88) and 0.91 (0.89-0.94), respectively. The overall AUC was 0.76 (0.74-0.79) for CA125, 0.81 (0.78-0.83) for HE4, 0.82 (0.80-0.85) for ROMA, 0.86 (0.84-0.88) for RMI I, 0.83 (0.81-0.86) for RMI IV, and 0.92 (0.90-0.94) for ADNEX. The NB for ADNEX was higher than other biomarkers and models across all decision thresholds between 5% and 50%.
Conclusions: The ADNEX model showed a better discrimination ability and clinical utility when differentiating malignant from benign Ams, compared to CA125, HE4, ROMA score, RMI I, and RMI IV.
Keywords: ADNEX; IOTA; ovarian cancer; serum markers; ultrasound.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Heintz A.P., Odicino F., Maisonneuve P., Quinn M.A., Benedet J.L., Creasman W.T., Ngan H.Y., Pecorelli S., Beller U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006;95((Suppl. S1)):S161–S192. - PubMed
-
- Froyman W., Landolfo C., De Cock B., Wynants L., Sladkevicius P., Testa A.C., Van Holsbeke C., Domali E., Fruscio R., Epstein E., et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): A 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019;20:448–458. doi: 10.1016/S1470-2045(18)30837-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

