Dose-Reduced FLA-IDA in Combination with Venetoclax Is an Effective and Safe Salvage Therapy in Relapsed and Refractory Acute Myeloid Leukemia (R/R AML)
- PMID: 39594827
- PMCID: PMC11592574
- DOI: 10.3390/cancers16223872
Dose-Reduced FLA-IDA in Combination with Venetoclax Is an Effective and Safe Salvage Therapy in Relapsed and Refractory Acute Myeloid Leukemia (R/R AML)
Abstract
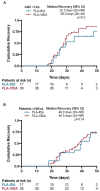
Despite the development of targeted therapies in first-line AML, complete remissions (CR) cannot be achieved in 30-40%, and relapse rates remain high. In R/R AML the intensive treatment regimen of fludarabine, cytarabine, idarubicin combined with venetoclax (FLA-VIDA) showed improved remission rates compared to FLA-IDA. In this retrospective single-center analysis, we investigated the efficacy and safety of dose-reduced FLA-IDA with and without venetoclax to minimize the risk of infectious complications and excessive myelosuppression; Methods: Between 2011 and 2023, 89 R/R AML patients were treated with dose-reduced FLA-IDA (fludarabine 30 mg/m2 day 1-4, cytarabine 2000 mg/m2 day 1-4, idarubicin 10 mg/m2 day 1 + 4). From 2019 onwards, venetoclax was added (day 1 100 mg, day 2 200 mg, day 3-14 400 mg); Results: Significantly improved response rates were observed with 60.0% vs. 38.8% CR/CRi (p = 0.0297) and 74.5% vs. 47.3% (p = 0.032) CR/CRi/MLFS for FLA-VIDA vs. FLA-IDA. Further, with FLA-VIDA significantly improved event-free survival (EFS) was observed (p = 0.026). Overall survival (OS) was similar in FLA-VIDA and FLA-IDA treated patients. The most common treatment-related toxicities were hematological adverse events, but they were comparable between groups. The time to neutrophil and platelet recovery were similar in responding patients treated with FLA-VIDA vs. FLA-IDA; Conclusions: Dose-reduced FLA-VIDA significantly improved response rates without increases in toxicity, showing promise for an improved R/R AML treatment.
Keywords: FLAG-IDA; acute myeloid leukemia; relapsed/refractory; venetoclax.
Conflict of interest statement
M.S. received support for meeting attendance from AMGEN. S.C. received support for meeting attendance from AbbVie and Jazz Pharmaceuticals. W.F. received personal fees and non-financial support from AbbVie; grants, personal fees, and non-financial support from Amgen and Pfizer; and personal fees from Jazz Pharmaceuticals, Celgene, MorphoSys, Ariad/Incyte, Stemline Therapeutics, Clinigen, Daiichi Sankyo, Otsuka and Servier outside the submitted work; in addition, research support from Apis; patent issued with Amgen; support for medical writing for Amgen, Pfizer, and AbbVie. C.B. received financial support from AOK Germany, Astra Zeneca, Bayer Healthcare, BioNTech, Bristol Myers Squipp, Jansen Cilag, med update, Merck Serono, Oncology Drug Consult CRO, Roche Pharma, Sanofi Aventis; and non-financial support from DGHO, Hamburg Cancer Society, National Network of German Cancer Centers, Northern German Society of Internal Medicine. The medical department of S.C., M.S., P.S., W.F. and C.B. are involved in several clinical trials sponsored by industry and cooperative groups where we hold participants roles and local PI roles and PI roles. M.H. received research funding from Abbvie, Servier, Astellas, BergenBio, Glycostem, Jazz Pharmaceuticals, Karyopharm, Loxo Oncology, Novartis, PinotBio. M.H. received personal fees and non-financial support from Abbvie, Bristol Myers Squibb, Janssen, Jazz Pharmaceuticals, Pfizer, Qiagen, Servier, Sobi; Consultancy: AvenCell, Abbvie, Astellas, Glycostem, Janssen, LabDelbert, Miltenyi, Novartis, Pfizer, PinotBio, Servier. F.A.A. received research funding from Mallinckrodt/Therakos. F.A.A. received personal fees and non-financial support from Novartis, Kite/Gilead, Janssen, BMS/Celgene, Mallinckrodt/Therakos, MiltenyiBiomedicine, Medac, AbbVie and Takeda. F.T. received personal fees and non-financial support from Abbvie and Astellas. Advisory board/consultancy: BMS, Novartis, Abbvie, Menarini, Rigel. All others declare no conflicts of interest relevant to this publication.
Figures




References
-
- Modemann F., Ghandili S., Oelrich J., Bokemeyer C., Fiedler W. Aktuelle Therapieoptionen bei rezidivierter/refraktärer AML. InFo Hämatol. Onkol. 2022;25:18–23. doi: 10.1007/s15004-022-9706-y. - DOI
LinkOut - more resources
Full Text Sources

