Bacterial Meningoencephalitis in Newborns
- PMID: 39595056
- PMCID: PMC11591924
- DOI: 10.3390/biomedicines12112490
Bacterial Meningoencephalitis in Newborns
Abstract
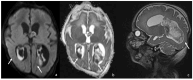
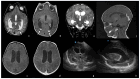
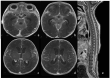
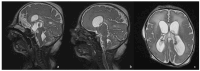
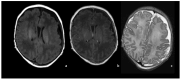
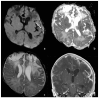
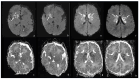
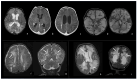
Bacterial meningoencephalitis in newborns is a severe and life-threatening pathology, which results from meningeal infection and the subsequent involvement of the brain parenchyma. The severity of the acute onset of symptoms and the risk of neurodevelopmental adverse sequelae in children strongly depend on the timing of the infection, the immunological protection transmitted by the mother to the fetus during pregnancy, and the neonate's inflammatory and immune system response after birth. Although the incidence of neonatal meningitis and meningoencephalitis and related mortality declined in the past twenty years with the improvement of prenatal care and with the introduction of intrapartum antibiotic prophylaxis against Streptococcus beta Hemolyticus group B (Streptococcus Agalactiae) in the 1990s, bacterial meningitis remains the most common form of cerebrospinal fluid infection in pediatric patients. To date, the rate of unfavorable neurological outcomes is still from 20% to 60%, and the possibility of containing its rate strongly depends on early diagnosis, therapy, and a multidisciplinary approach, which involves neonatologists, neurologists, neuroradiologists, and physiotherapists. Neonatal meningitis remains difficult to diagnose because the responsible bacteria vary with gestational age at birth, age at presentation, and environmental context. The clinical presentation, especially in the newborn, is very ambiguous. From a clinical point of view, the definitive test for diagnosis is lumbar puncture in patients with symptoms suggestive of neurological involvement. Therefore, neuroimaging is key for raising clinical suspicion of meningitis or corroborating the diagnosis based on clinical and laboratory data. Our pictorial review offers a practical approach to neonatal meningoencephalitis by describing the epidemiology, the pathophysiology of bacterial meningoencephalitis, defining the indications and suggesting optimized protocols for neuroimaging techniques, and showing the main neuroimaging findings to reach the diagnosis and offering proper follow-up of bacterial meningitis. Moreover, we tried identifying some peculiar MRI patterns related to some bacteria.
Keywords: CT; MRI; US; bacterial meningoencephalitis; encephalitis; meningitis; meningoencephalitis; newborns; pediatric.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Moltoni G., Lucignani G., Sgrò S., Guarnera A., Rossi Espagnet M.C., Dellepiane F., Carducci C., Liberi S., Iacoella E., Evangelisti G., et al. MRI Scan with the “Feed and Wrap” Technique and with an Optimized Anesthesia Protocol: A Retrospective Analysis of a Single-Center Experience. Front. Pediatr. 2024;12:1415603. doi: 10.3389/fped.2024.1415603. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

