Uricase-Expressing Engineered Macrophages Alleviate Murine Hyperuricemia
- PMID: 39595167
- PMCID: PMC11592275
- DOI: 10.3390/biomedicines12112602
Uricase-Expressing Engineered Macrophages Alleviate Murine Hyperuricemia
Abstract
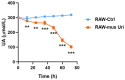
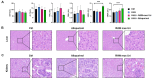
Background: Uricase, or urate oxidase (Uox) is a key enzyme in uric acid (UA) metabolism and has been applied in clinical treatment of human hyperuricemia (HUA). However, the current clinically applied uricases, despite their potent urate-lowering capacity, tend to form anti-drug antibodies because of their immunogenicity, leading to increased risk of anaphylaxis, faster drug clearance and reduced or even complete loss of therapeutic effect, limiting their clinical application. In this study, we constructed engineered macrophages that stably expressed uricase, which might serve as a promising alternative to the direct injection of uricases. Materials and Methods: Engineered macrophages RAW264.7 cells were injected intravenously to treat hyperuricemic KM mice. Serum uric acid and bio-indicators for renal and hepatic functions were detected by an automatic biochemical analyzer; inflammatory cytokines were determined by ELISA; the livers and kidneys of the mice were sectioned for histological examination. Results: The uricase-expressing macrophages reduced UA levels from 300 ± 1.5 μmol/L to 101 ± 8.3 μmol/L in vitro. And in an HUA mouse model established by gavage with yeast extract, intravenous injection of the engineered macrophages could reduce the serum uric acid (sUA) of mice to normal level on the 14th day of modeling, with a decrease of 48.6%, and the urate-lowering effect was comparable to that of the first-line clinical drug allopurinol. In terms of safety, engineered macrophages did not cause liver or kidney dysfunction in mice, nor did they induce systemic immune response. Conclusions: Using macrophages as a chassis to deliver uricase might be a new, safe and effective strategy for the treatment and control of hyperuricemia.
Keywords: engineered macrophages; hyperuricemia; macrophage therapy; uricase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Taylor W.J., Fransen J., Jansen T.L., Dalbeth N., Schumacher H.R., Brown M., Louthrenoo W., Vazquez-Mellado J., Eliseev M., McCarthy G., et al. Study for Updated Gout Classification Criteria: Identification of Features to Classify Gout. Arthritis Care Res. 2015;67:1304–1315. doi: 10.1002/acr.22585. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

