Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer
- PMID: 39595190
- PMCID: PMC11592003
- DOI: 10.3390/biomedicines12112625
Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer
Abstract
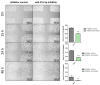
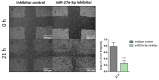
Background: MicroRNAs are well established as master regulators of carcinogenesis and potential biomarkers in breast cancer (BC). In a preliminary effort, we found miR-27a-5p to be significantly downregulated in experimentally derived mammospheres and BC patients from The Cancer Genome Atlas Breast Invasive Carcinoma (TCGA-BRCA) dataset. Objectives. Herein, we sought to investigate the putative involvement of miR-27a-5p in promoting a migratory phenotype of breast cancer cells, and establish whether miR-27a-5p is associated with patient clinicopathological characteristics. Methods: miR-27a-5p capability of inducing a metastasis-prone cell phenotype was analyzed in SUM159 and MDA-MB-231, both representing the triple negative BC subtype. miR-27a-5p expression profile was carried out in a cohort of 232 BC patients and normal breast tissues (NBTs) by RT-qPCR. Results: Transient miR-27a-5p inhibition did not affect cell proliferation but led to a significant increase of cell migration in knocked-down compared to control cells. Following quantification in the patient cohort, miR-27a-5p was found higher in NBTs (Median 2.28, IQR 1.50-5.40) and pre-invasive breast lesions (Median 3.32, IQR 1.68-4.32) compared to tumors. In particular, miR-27a-5p was less expressed in patients with synchronous (Median 1.03, IQR 0.83-1.58) or metachronous (Median 1.83, IQR 1.29-3.17) metastases than in patients free from metastases after a 5-year follow-up (Median 2.17, IQR 1.19-3.64), suggesting that miR-27a-5p expression is negatively correlated with breast pathology evolution (R = -0.13, p = 0.038). However, time-to-event analysis did not highlight significant associations with patient outcome in either our internal cohort or TCGA-BRCA dataset. Conclusions: Our study suggests a potential role of miR-27a-5p as tumor suppressor miRNA in breast cancer. Further investigations may help define its biomarker potential in each breast cancer subtype, and identify other molecular partners as targets for new interventions.
Keywords: breast cancer; metastases; miR-27a-5p; microRNA; prognosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Benitez Fuentes J.D., Morgan E., de Luna Aguilar A., Mafra A., Shah R., Giusti F., Vignat J., Znaor A., Musetti C., Yip C.-H., et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024;10:71–78. doi: 10.1001/jamaoncol.2023.4837. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

