Pyroptosis: An Accomplice in the Induction of Multisystem Complications Triggered by Obstructive Sleep Apnea
- PMID: 39595526
- PMCID: PMC11592050
- DOI: 10.3390/biom14111349
Pyroptosis: An Accomplice in the Induction of Multisystem Complications Triggered by Obstructive Sleep Apnea
Abstract
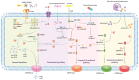
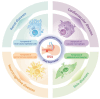
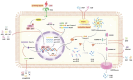
Obstructive sleep apnea (OSA) is a common respiratory disorder, primarily characterized by two pathological features: chronic intermittent hypoxia (CIH) and sleep deprivation (SD). OSA has been identified as a risk factor for numerous diseases, and the inflammatory response related to programmed cell necrosis is believed to play a significant role in the occurrence and progression of multisystem damage induced by OSA, with increasing attention being paid to pyroptosis. Recent studies have indicated that OSA can elevate oxidative stress levels in the body, activating the process of pyroptosis within different tissues, ultimately accelerating organ dysfunction. However, the molecular mechanisms of pyroptosis in the multisystem damage induced by OSA remain unclear. Therefore, this review focuses on four major systems that have received concentrated attention in existing research in order to explore the role of pyroptosis in promoting renal diseases, cardiovascular diseases, neurocognitive diseases, and skin diseases in OSA patients. Furthermore, we provide a comprehensive overview of methods for inhibiting pyroptosis at different molecular levels, with the goal of identifying viable targets and therapeutic strategies for addressing OSA-related complications.
Keywords: NLRP3 inflammasome; chronic intermittent hypoxia; obstructive sleep apnea; pyroptosis; sleep deprivation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
TRPC5-mediated NLRP3 inflammasome activation contributes to myocardial cell pyroptosis in chronic intermittent hypoxia rats.Acta Cardiol. 2024 Sep;79(7):796-804. doi: 10.1080/00015385.2024.2408137. Epub 2024 Oct 8. Acta Cardiol. 2024. PMID: 39377158
-
Sex-dependent effects of chronic intermittent hypoxia: implication for obstructive sleep apnea.Biol Sex Differ. 2024 Apr 25;15(1):38. doi: 10.1186/s13293-024-00613-3. Biol Sex Differ. 2024. PMID: 38664845 Free PMC article.
-
Chronic intermittent hypoxia induces the pyroptosis of renal tubular epithelial cells by activating the NLRP3 inflammasome.Bioengineered. 2022 Mar;13(3):7528-7540. doi: 10.1080/21655979.2022.2047394. Bioengineered. 2022. PMID: 35263214 Free PMC article.
-
Cardiovascular Disorders Triggered by Obstructive Sleep Apnea-A Focus on Endothelium and Blood Components.Int J Mol Sci. 2021 May 12;22(10):5139. doi: 10.3390/ijms22105139. Int J Mol Sci. 2021. PMID: 34066288 Free PMC article. Review.
-
Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications.Antioxid Redox Signal. 2008 Apr;10(4):755-68. doi: 10.1089/ars.2007.1946. Antioxid Redox Signal. 2008. PMID: 18177236 Review.
References
-
- Qian L., Rawashdeh O., Kasas L., Milne M.R., Garner N., Sankorrakul K., Marks N., Dean M.W., Kim P.R., Sharma A., et al. Cholinergic basal forebrain degeneration due to sleep-disordered breathing exacerbates pathology in a mouse model of Alzheimer’s disease. Nat. Commun. 2022;13:6543. doi: 10.1038/s41467-022-33624-y. - DOI - PMC - PubMed
-
- Liu W., Zhao D., Wu X., Yue F., Yang H., Hu K. Rapamycin ameliorates chronic intermittent hypoxia and sleep deprivation-induced renal damage via the mammalian target of rapamycin (mTOR)/NOD-like receptor protein 3 (NLRP3) signaling pathway. Bioengineered. 2022;13:5537–5550. doi: 10.1080/21655979.2022.2037872. - DOI - PMC - PubMed
-
- Korkalainen H., Kainulainen S., Islind A.S., Óskarsdóttir M., Strassberger C., Nikkonen S., Töyräs J., Kulkas A., Grote L., Hedner J., et al. Review and perspective on sleep-disordered breathing research and translation to clinics. Sleep Med. Rev. 2024;73:101874. doi: 10.1016/j.smrv.2023.101874. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

