Genetic Engineering Approaches for the Microbial Production of Vanillin
- PMID: 39595589
- PMCID: PMC11591617
- DOI: 10.3390/biom14111413
Genetic Engineering Approaches for the Microbial Production of Vanillin
Abstract
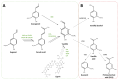
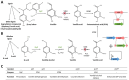
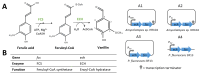
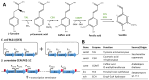
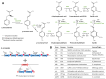
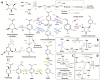
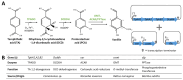
Vanilla flavour is widely used in various industries and is the most broadly used flavouring agent in the food industry. The demand for this flavour is, therefore, extremely high, yet vanilla bean extracts can only meet about 1% of the overall demand. Vanillin, the main constituent of vanilla flavour, can easily be obtained through chemical synthesis. Nonetheless, consumer demands for natural products and environmentally friendly industrial processes drive the development of biotechnological approaches for its production. Some microorganisms can naturally produce vanillin when fed with various substrates, including eugenol, isoeugenol, and ferulic acid. The characterisation of the genes and enzymes involved in these bioconversion pathways, as well as progress in the understanding of vanillin biosynthesis in Vanilla orchids, allowed the development of genetic engineering and synthetic biology approaches to increase vanillin production in naturally vanillin-producing microorganisms, or to implement novel vanillin biosynthetic pathways in microbial chassis. This review summarises and discusses these genetic engineering and synthetic biology approaches for the microbial production of vanillin.
Keywords: accessible raw material; bioconversion; metabolic engineering; microbial production; pathway design; synthetic biology; vanillin.
Conflict of interest statement
J.-L.P. is an inventor in the patent “Bacterial Strains for the Production of Vanillin” [71]. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Martău G.A., Călinoiu L.F., Vodnar D.C. Bio-Vanillin: Towards a Sustainable Industrial Production. Trends Food Sci. Technol. 2021;109:579–592. doi: 10.1016/j.tifs.2021.01.059. - DOI
-
- Korthou H., Verpoorte R. Vanilla. In: Berger R.G., editor. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Springer; Hannover, Germany: 2007. pp. 203–217.
-
- GVR . Vanillin Market Size, Share|Global Industry Report, 2018–2025. Grand View Research; San Francisco, CA, USA: 2017.
-
- PMR . Bio Vanillin Market. Persistence Market Research; Maharashtra, India: 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

