Chaga Mushroom Triterpenoids Inhibit Dihydrofolate Reductase and Act Synergistically with Conventional Therapies in Breast Cancer
- PMID: 39595631
- PMCID: PMC11591880
- DOI: 10.3390/biom14111454
Chaga Mushroom Triterpenoids Inhibit Dihydrofolate Reductase and Act Synergistically with Conventional Therapies in Breast Cancer
Abstract
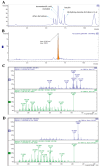
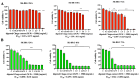
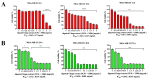
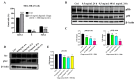
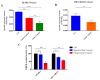
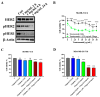
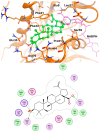
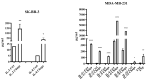
Inonotus obliquus (Chaga) is a medicinal mushroom with several pharmacological properties that is used as a tea in traditional Chinese medicine. In this study, Chaga water extract was digested in vitro to mimic the natural processing and absorption of its biocomponents when it is consumed as functional beverage, and its anticancer activities were evaluated in breast cancer (BC) cell lines, representing HER2-positive and triple-negative subtypes. After chemical characterization by liquid chromatography/mass spectrometry (HR-QTOF) analysis, the effect of Chaga biocomponents on cell viability and cell cycle progression was assessed by MTT assay, FACS analysis, and Western blot. Dihydrofolate reductase (DHFR) activity was measured by an enzymatic assay. Four highly bioactive triterpenoids (inotodiol, trametenolic acid, 3-hydroxy-lanosta-8,24-dien-21-al, and betulin) were identified as the main components, able to decrease BC cell viability and block the cell cycle in G0/G1 by inducing the downregulation of cyclin D1, CDK4, cyclin E, and phosphorylated retinoblastoma protein. DHFR was identified as their crucial target. Moreover, bioactive Chaga components exerted a synergistic action with cisplatin and with trastuzumab in SK-BR-3 cells by inhibiting both HER2 and HER1 activation and displayed an immunomodulatory effect. Thus, Inonotus obliquus represents a source of triterpenoids that are effective against aggressive BC subtypes and display properties of targeted drugs.
Keywords: Chaga mushroom (Inonotus obliquus); DHFR; breast cancer; cell cycle regulation; triterpenoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Eisen M.B., Hastie T., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad.Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. - DOI - PMC - PubMed
-
- Millar E.K., Graham P.H., O’Toole S.A., McNeil C.M., Browne L., Morey A.L., Eggleton S., Beretov J., Theocharous C., Capp A., et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J. Clin. Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

