Tumor-Colonizing E. coli Expressing Both Collagenase and Hyaluronidase Enhances Therapeutic Efficacy of Gemcitabine in Pancreatic Cancer Models
- PMID: 39595636
- PMCID: PMC11591662
- DOI: 10.3390/biom14111458
Tumor-Colonizing E. coli Expressing Both Collagenase and Hyaluronidase Enhances Therapeutic Efficacy of Gemcitabine in Pancreatic Cancer Models
Abstract
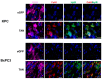
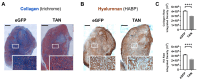
Desmoplasia is a hallmark feature of pancreatic ductal adenocarcinoma (PDAC) that contributes significantly to treatment resistance. Approaches to enhance drug delivery into fibrotic PDAC tumors continue to be an important unmet need. In this study, we have engineered a tumor-colonizing E. coli-based agent that expresses both collagenase and hyaluronidase as a strategy to reduce desmoplasia and enhance the intratumoral perfusion of anticancer agents. Overall, we observed that the tandem expression of both these enzymes by tumor-colonizing E. coli resulted in the reduced presence of intratumoral collagen and hyaluronan, which likely contributed to the enhanced chemotherapeutic efficacy observed when used in combination. These results highlight the importance of combination treatments involving the depletion of desmoplastic components in PDAC before or during treatment.
Keywords: bacterial vector; collagen; collagenase; desmoplasia; fibrosis; hyaluronan; hyaluronidase; pancreatic ductal adenocarcinoma; resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Kang J., Hwang I., Yoo C., Kim K.P., Jeong J.H., Chang H.M., Lee S.S., Park D.H., Song T.J., Seo D.W., et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: Retrospective analysis. Investig. New Drugs. 2018;36:732–741. doi: 10.1007/s10637-018-0598-5. - DOI - PubMed
-
- Hwang H.J., Oh M.S., Lee D.W., Kuh H.J. Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J. Exp. Clin. Cancer Res. 2019;38:258. doi: 10.1186/s13046-019-1225-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

