DLK1 Is Associated with Stemness Phenotype in Medullary Thyroid Carcinoma Cell Lines
- PMID: 39595993
- PMCID: PMC11594232
- DOI: 10.3390/ijms252211924
DLK1 Is Associated with Stemness Phenotype in Medullary Thyroid Carcinoma Cell Lines
Abstract
Medullary thyroid carcinoma (MTC) is a rare and aggressive tumor, often requiring systemic treatment in advanced or metastatic stages, where drug resistance presents a significant challenge. Given the role of cancer stem cells (CSCs) in cancer recurrence and drug resistance, we aimed to identify CSC subpopulations within two MTC cell lines harboring pathogenic variants in the two most common MEN2-associated codons. We analyzed 15 stemness-associated markers, along with well-established thyroid stem cell markers (CD133, CD44, and ALDH1), a novel candidate (DLK1), and multidrug resistance proteins (MRP1 and MRP3). The ability to efflux the fluorescent dye Hoechst 3342 and form spheroids, representing CSC behavior, was also assessed. MZ-CRC-1 cells (p.M918T) displayed higher expressions of canonical markers, DLK1, and MRP proteins than TT cells (p.C634W). MZ-CRC-1 cells also formed more spheroids and showed less dye accumulation (p < 0.0001). Finally, we observed that DLK1+ cells (those expressing DLK1) in both cell lines exhibited significantly higher levels of stemness markers compared to DLK1- cells (those lacking DLK1 expression). These findings underscore DLK1's role in enhancing the stemness phenotype, providing valuable insights into MTC progression and resistance and suggesting potential therapeutic implications.
Keywords: CD133; CD44; DLK1; RET; cancer stem cells; medullary thyroid carcinoma; p.M918T.
Conflict of interest statement
The authors declare that no conflicts of interest could be perceived as prejudicing the impartiality of the research reported.
Figures

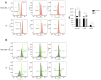
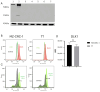


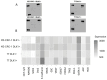

References
-
- American Thyroid Association Guidelines Task Force. Kloos R.T., Eng C., Evans D.B., Francis G.L., Gagel R.F., Gharib H., Moley J.F., Pacini F., Ringel M.D., et al. Medullary thyroid cancer: Management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. Erratum in Thyroid 2009, 19, 1295. - DOI - PubMed
-
- Araujo A.N., Moraes L., França M.I., Hakonarson H., Li J., Pellegrino R., Maciel R.M., Cerutti J.M. Genome-wide copy number analysis in a family with p.G533C RET mutation and medullary thyroid carcinoma identified regions potentially associated with a higher predisposition to lymph node metastasis. J. Clin. Endocrinol. Metab. 2014;99:E1104–E1112. doi: 10.1210/jc.2013-2993. - DOI - PubMed
-
- Signorini P.S., França M.I., Camacho C.P., Lindsey S.C., Valente F.O., Kasamatsu T.S., Machado A.L., Salim C.P., Delcelo R., Hoff A.O., et al. A ten-year clinical update of a large RET p.Gly533Cys kindred with medullary thyroid carcinoma emphasizes the need for an individualized assessment of affected relatives. Clin. Endocrinol. 2014;80:235–245. doi: 10.1111/cen.12264. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

