Alzheimer's Disease as Type 3 Diabetes: Understanding the Link and Implications
- PMID: 39596023
- PMCID: PMC11593477
- DOI: 10.3390/ijms252211955
Alzheimer's Disease as Type 3 Diabetes: Understanding the Link and Implications
Abstract
Alzheimer's disease (AD) and type 2 diabetes mellitus (T2DM) are two prevalent conditions that present considerable public health issue in aging populations worldwide. Recent research has proposed a novel conceptualization of AD as "type 3 diabetes", highlighting the critical roles of insulin resistance and impaired glucose metabolism in the pathogenesis of the disease. This article examines the implications of this association, exploring potential new avenues for treatment and preventive strategies for AD. Key evidence linking diabetes to AD emphasizes critical metabolic processes that contribute to neurodegeneration, including inflammation, oxidative stress, and alterations in insulin signaling pathways. By framing AD within this metabolic context, we can enhance our understanding of its etiology, which in turn may influence early diagnosis, treatment plans, and preventive measures. Understanding AD as a manifestation of diabetes opens up the possibility of employing novel therapeutic strategies that incorporate lifestyle modifications and the use of antidiabetic medications to mitigate cognitive decline. This integrated approach has the potential to improve patient outcomes and deepen our comprehension of the intricate relationship between neurodegenerative diseases and metabolic disorders.
Keywords: Alzheimer’s disease; glucose metabolism; neurodegeneration; public health; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
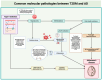

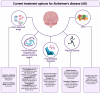
References
-
- Andrade-Guerrero J., Santiago-Balmaseda A., Jeronimo-Aguilar P., Vargas-Rodríguez I., Cadena-Suárez A.R., Sánchez-Garibay C., Pozo-Molina G., Méndez-Catalá C.F., Cardenas-Aguayo M.-D.-C., Diaz-Cintra S., et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023;24:3754. doi: 10.3390/ijms24043754. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

