A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome
- PMID: 39596084
- PMCID: PMC11593901
- DOI: 10.3390/ijms252212015
A Small Intestinal Helminth Infection Alters Colonic Mucus and Shapes the Colonic Mucus Microbiome
Abstract
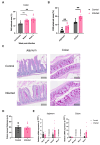
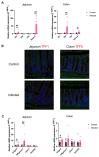
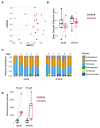
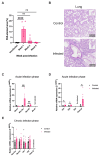
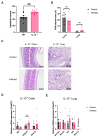
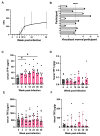
Infecting humans with controlled doses of small intestinal helminths, such as human hookworm, is proposed as a therapy for the colonic inflammatory disease ulcerative colitis. Strengthening the colonic mucus barrier is a potential mechanism by which small intestinal helminths could treat ulcerative colitis. In this study, we compare C57BL/6 mice infected with the small intestinal helminth Heligmosomoides polygyrus and uninfected controls to investigate changes in colonic mucus. Histology, gene expression, and immunofluorescent analysis demonstrate that this helminth induces goblet cell hyperplasia, and an upregulation of mucin sialylation, and goblet-cell-derived functional proteins resistin-like molecule-beta (RELM-β) and trefoil factors (TFFs), in the colon. Using IL-13 knockout mice, we reveal that these changes are predominantly IL-13-dependent. The assessment of the colonic mucus microbiome demonstrates that H. polygyrus infection increases the abundance of Ruminococcus gnavus, a commensal bacterium capable of utilising sialic acid as an energy source. This study also investigates a human cohort experimentally challenged with human hookworm. It demonstrates that TFF blood levels increase in individuals chronically infected with small intestinal helminths, highlighting a conserved mucus response between humans and mice. Overall, small intestinal helminths modify colonic mucus, highlighting this as a plausible mechanism by which human hookworm therapy could treat ulcerative colitis.
Keywords: helminth; hookworm; intestinal barrier; microbiome; mucus; trefoil factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Mules T.C., Lavender B., Maclean K., Vacca F., Noble S.-L., Yumnam B., Te Kawa T., Cait A., Tang J., O’Sullivan D., et al. Controlled Hookworm Infection for Medication-Free Maintenance in Patients with Ulcerative Colitis: A Pilot, Double-Blind, Randomized Control Trial. Inflamm. Bowel Dis. 2023;30:735–745. doi: 10.1093/ibd/izad110. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

