The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer
- PMID: 39596123
- PMCID: PMC11593775
- DOI: 10.3390/ijms252212054
The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer
Abstract
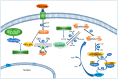
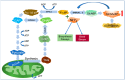
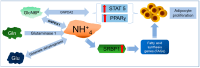
Nearly 5% of the glucose-6-phosphate (Glc6P) in cells is diverted into the hexosamine biosynthetic pathway (HBP) to synthesize glucosamine-6-phosphate (GlcN6P) and uridine diphosphate N-acetyl-glucosamine-6-phosphate (UDP-GlcN6P). Fructose-6-phosphate (Fru6P) is a common intermediary between glycolysis and the HBP. Changes in HBP regulation cause abnormal protein N-glycosylation and O-linked-N-acetylglucosamine modification (O-GlcNAcylation), affecting protein function and modifying cellular responses to signals. The HBP enzymes glucosamine-6-phosphate deaminases 1 and 2 (GNPDA1 and 2) turn GlcN6P back into Fru6P and ammonium, and have been implicated in cancer and metabolic diseases. Despite the plentiful literature on this topic, the mechanisms involved are just beginning to be studied. In this review, we summarize, for the first time, the current knowledge regarding the possible roles of the isoenzymes of both GNPDAs in the pathogenesis and development of metabolic diseases and cancer from a molecular point of view, highlighting their importance not only in supplying carbon from glycolysis, but also in ammonia metabolism.
Keywords: ammonium; cancer; glucosamine-6-phosphate deaminases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

