Human-Brain-Derived Ischemia-Induced Stem Cell Transplantation Is Associated with a Greater Neurological Functional Improvement Compared with Human-Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Mice After Stroke
- PMID: 39596134
- PMCID: PMC11593343
- DOI: 10.3390/ijms252212065
Human-Brain-Derived Ischemia-Induced Stem Cell Transplantation Is Associated with a Greater Neurological Functional Improvement Compared with Human-Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Mice After Stroke
Abstract
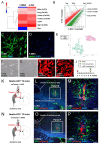
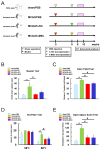
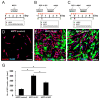
The transplantation of injury/ischemia-induced stem cells (iSCs) extracted from post-stroke human brains can improve the neurological functions of mice after stroke. However, the usefulness of iSCs as an alternative stem cell source remains unclear. The current study aimed to assess the efficacy of iSC and mesenchymal stem cell (MSC) transplantation. In this experiment, equal numbers of human brain-derived iSCs (h-iSCs) (5.0 × 104 cells/μL) and human bone marrow-derived MSCs (h-MSCs) (5.0 × 104 cells/μL) were intracranially transplanted into post-stroke mouse brains after middle cerebral artery occlusion. Results showed that not only h-iSC transplantation but also h-MSC transplantation activated endogenous neural stem/progenitor cells (NSPCs) around the grafted sites and promoted neurological functional improvement. However, mice that received h-iSC transplantation experienced improvement in a higher number of behavioral tasks compared with those that received h-MSC transplantation. To investigate the underlying mechanism, NSPCs extracted from the ischemic areas of post-stroke mouse brains were cocultured with h-iSCs or h-MSCs. After coincubation, NSPCs, h-iSCs, and h-MSCs were selectively collected via fluorescence-activated cell sorting. Next, their traits were analyzed via microarray analysis. The genes related to various neuronal lineages in NSPCs after coincubation with h-iSCs were enriched compared with those in NSPCs after coincubation with h-MSCs. In addition, the gene expression patterns of h-iSCs relative to those of h-MSCs showed that the expression of genes related to synapse formation and neurotransmitter-producing neurons increased more after coincubation with NSPCs. Hence, cell-cell interactions with NSPCs promoted transdifferentiation toward functional neurons predominantly in h-iSCs. In accordance with these findings, immunohistochemistry showed that the number of neuronal networks between NSPCs and h-iSCs was higher than that between NSPCs and h-MSCs. Therefore, compared with h-MSC transplantation, h-iSC transplantation is associated with a higher neurological functional improvement, presumably by more effectively modulating the fates of endogenous NSPCs and grafted h-iSCs themselves.
Keywords: cell transplantation; injury/ischemia-induced stem cells; ischemic stroke; mesenchymal stem cells; neural stem/progenitor cells; neurological function.
Conflict of interest statement
The Department of Therapeutic Progress in Brain Diseases is financially supported by Nippon Zoki Pharmaceutical Co., Ltd. and CLEA Japan, Inc. The sponsors had no role in the work, including study design, data collection, data analysis, data interpretation, and manuscript writing.
Figures







References
-
- Doi D., Samata B., Katsukawa M., Kikuchi T., Morizane A., Ono Y., Sekiguchi K., Nakagawa M., Parmar M., Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. - DOI - PMC - PubMed
-
- Vats K., Sarmah D., Datta A., Saraf J., Kaur H., Pravalika K., Wanve M., Kalia K., Borah A., Dave K.R., et al. Intra-arterial Stem Cell Therapy Diminishes Inflammasome Activation After Ischemic Stroke: A Possible Role of Acid Sensing Ion Channel 1a. J. Mol. Neurosci. 2021;71:419–426. doi: 10.1007/s12031-019-01460-3. - DOI - PubMed
-
- Kikuchi-Taura A., Okinaka Y., Takeuchi Y., Ogawa Y., Maeda M., Kataoka Y., Yasui T., Kimura T., Gul S., Claussen C., et al. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction-Mediated Cell-Cell Interaction. Stroke. 2020;51:1279–1289. doi: 10.1161/STROKEAHA.119.028072. - DOI - PubMed
-
- Nakagomi T., Taguchi A., Fujimori Y., Saino O., Nakano-Doi A., Kubo S., Gotoh A., Soma T., Yoshikawa H., Nishizaki T., et al. Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur. J. Neurosci. 2009;29:1842–1852. doi: 10.1111/j.1460-9568.2009.06732.x. - DOI - PubMed
MeSH terms
Grants and funding
- 15K06723/Japan Society for the Promotion of Science (JSPS) KAKENHI
- 18K07380/Japan Society for the Promotion of Science (JSPS) KAKENHI
- 21nk0101538h0002/Japan Agency for Medical Research and Development (AMED)
- None/the Grant-in-Aid from the Japanese Society of Cerebral Blood Flow and Metabolism (2019)
- None/Hyogo Innovative Challenge (2022)
LinkOut - more resources
Full Text Sources
Medical

