Inert Gas Mild Pressure Action on Healthy Humans: The "IPA" Study
- PMID: 39596136
- PMCID: PMC11593890
- DOI: 10.3390/ijms252212067
Inert Gas Mild Pressure Action on Healthy Humans: The "IPA" Study
Abstract
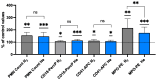
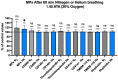
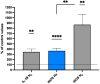
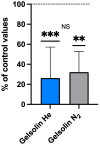
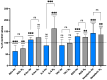
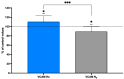
The goal of this study was to evaluate inflammatory and oxidative stress responses in human subjects (9 females and 15 males) (age [29.6 ± 11.5 years old (mean ± SD)], height [172.0 ± 10.05 cm], and weight [67.8 ± 12.4 kg]) exposed to 1.45 ATA of helium (He) or nitrogen (N2) without concurrent hyperoxia. We hypothesized that elevated gas pressures would elicit an inflammatory response concurrent with oxidative stress. Consistent with ex vivo studies, both gasses elicited neutrophil activation, small elevations in microparticles (MPs) and increases in intra-MP interleukin (IL)-1β and inflammatory nitric oxide synthase, and an increase in urinary IL-6 concurrent with a marked reduction in plasma gelsolin. Mixed responses indictive of oxidative stress, with some biomarker elevations but little change in others and a decrease in some, were observed. Overall, these results demonstrate that exposure to typical diving gasses at a mildly elevated partial pressure will initiate inflammatory responses, which may play a significant role in decompression sickness (DCS). The complex pattern of oxidative stress responses may be indicative of competing systemic reactions and sampling different body fluids.
Keywords: ROS; decompression sickness; diving; exosomes; extracellular vesicles; filamentous actin; inert gas; interleukin-1β; interleukin-6; microglia; microparticles; oxidative stress; oxyinflammation; plasma gelsolin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Microparticle and interleukin-1β production with human simulated compressed air diving.Sci Rep. 2019 Sep 16;9(1):13320. doi: 10.1038/s41598-019-49924-1. Sci Rep. 2019. PMID: 31527725 Free PMC article.
-
Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers.Int J Mol Sci. 2023 Mar 22;24(6):5969. doi: 10.3390/ijms24065969. Int J Mol Sci. 2023. PMID: 36983042 Free PMC article.
-
Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression.J Appl Physiol (1985). 2021 May 1;130(5):1604-1613. doi: 10.1152/japplphysiol.01062.2020. Epub 2021 Mar 25. J Appl Physiol (1985). 2021. PMID: 33764168 Free PMC article.
-
Recreational technical diving part 2: decompression from deep technical dives.Diving Hyperb Med. 2013 Jun;43(2):96-104. Diving Hyperb Med. 2013. PMID: 23813463 Review.
-
Pathophysiology of inner ear decompression sickness: potential role of the persistent foramen ovale.Diving Hyperb Med. 2015 Jun;45(2):105-10. Diving Hyperb Med. 2015. PMID: 26165533 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

