Unraveling Macrophage Polarization: Functions, Mechanisms, and "Double-Edged Sword" Roles in Host Antiviral Immune Responses
- PMID: 39596148
- PMCID: PMC11593441
- DOI: 10.3390/ijms252212078
Unraveling Macrophage Polarization: Functions, Mechanisms, and "Double-Edged Sword" Roles in Host Antiviral Immune Responses
Abstract
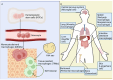
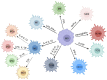
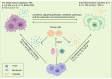
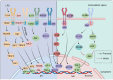
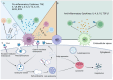
Numerous viruses that propagate through the respiratory tract may be initially engulfed by macrophages (Mφs) within the alveoli, where they complete their first replication cycle and subsequently infect the adjacent epithelial cells. This process can lead to significant pathological damage to tissues and organs, leading to various diseases. As essential components in host antiviral immune systems, Mφs can be polarized into pro-inflammatory M1 Mφs or anti-inflammatory M2 Mφs, a process involving multiple signaling pathways and molecular mechanisms that yield diverse phenotypic and functional features in response to various stimuli. In general, when infected by a virus, M1 macrophages secrete pro-inflammatory cytokines to play an antiviral role, while M2 macrophages play an anti-inflammatory role to promote the replication of the virus. However, recent studies have shown that some viruses may exhibit the opposite trend. Viruses have evolved various strategies to disrupt Mφ polarization for efficient replication and transmission. Notably, various factors, such as mechanical softness, the altered pH value of the endolysosomal system, and the homeostasis between M1/M2 Mφs populations, contribute to crucial events in the viral replication cycle. Here, we summarize the regulation of Mφ polarization, virus-induced alterations in Mφ polarization, and the antiviral mechanisms associated with these changes. Collectively, this review provides insights into recent advances regarding Mφ polarization in host antiviral immune responses, which will contribute to the development of precise prevention strategies as well as management approaches to disease incidence and transmission.
Keywords: antiviral immunity; immune escape; macrophage polarization; macrophages; viruses.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

