Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression
- PMID: 39596150
- PMCID: PMC11594236
- DOI: 10.3390/ijms252212082
Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression
Abstract
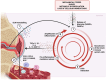
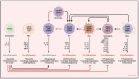
Osteoarthritis (OA) is a chronic progressive disease of the joint. Although representing the most frequent cause of disability in the elderly, OA remains partly obscure in its pathogenic mechanisms and is still the orphan of resolutive therapies. The concept of what was once considered a "wear and tear" of articular cartilage is now that of an inflammation-related disease that affects over time the whole joint. The attention is increasingly focused on the synovium. Even from the earliest clinical stages, synovial inflammation (or synovitis) is a crucial factor involved in OA progression and a major player in pain onset. The release of inflammatory molecules in the synovium mediates disease progression and worsening of clinical features. The activation of synovial tissue-resident cells recalls innate immunity cells from the bloodstream, creating a proinflammatory milieu that fuels and maintains a damaging condition of low-grade inflammation in the joint. In such a context, cellular and molecular inflammatory behaviors in the synovium could be the primum movens of the structural and functional alterations of the whole joint. This paper focuses on and discusses the involvement of innate immunity cells in synovitis and their role in the progression of OA.
Keywords: inflammaging; inflammation; innate immunity; innate immunity cells; loss of chondrocyte homeostasis; low-grade inflammation; osteoarthritis; osteoarthritis pathogenesis; synovitis; synovium.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

