An Immune-Independent Mode of Action of Tacrolimus in Promoting Human Extravillous Trophoblast Migration Involves Intracellular Calcium Release and F-Actin Cytoskeletal Reorganization
- PMID: 39596157
- PMCID: PMC11593602
- DOI: 10.3390/ijms252212090
An Immune-Independent Mode of Action of Tacrolimus in Promoting Human Extravillous Trophoblast Migration Involves Intracellular Calcium Release and F-Actin Cytoskeletal Reorganization
Abstract
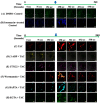
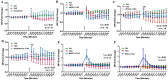
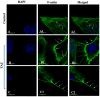
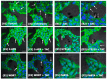
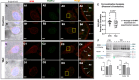
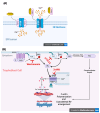
We have previously reported that the calcineurin inhibitor macrolide immunosuppressant Tacrolimus (TAC, FK506) can promote the migration and invasion of the human-derived extravillous trophoblast cells conducive to preventing implantation failure in immune-complicated gestations manifesting recurrent implantation failure. Although the exact mode of action of TAC in promoting implantation has yet to be elucidated, the integral association of its binding protein FKBP12 with the inositol triphosphate receptor (IP3R) regulated intracellular calcium [Ca2+]i channels in the endoplasmic reticulum (ER), suggesting that TAC can mediate its action through ER release of [Ca2+]i. Using the immortalized human-derived first-trimester extravillous trophoblast cells HTR8/SVneo, our data indicated that TAC can increase [Ca2+]I, as measured by fluorescent live-cell imaging using Fluo-4. Concomitantly, the treatment of HTR8/SVneo with TAC resulted in a major dynamic reorganization in the actin cytoskeleton, favoring a predominant distribution of cortical F-actin networks in these trophoblasts. Notably, the findings that TAC was unable to recover [Ca2+]i in the presence of the IP3R inhibitor 2-APB indicate that this receptor may play a crucial role in the mechanism of action of TAC. Taken together, our results suggest that TAC has the potential to influence trophoblast migration through downstream [Ca2+]i-mediated intracellular events and mechanisms involved in trophoblast migration, such as F-actin redistribution. Further research into the mono-therapeutic use of TAC in promoting trophoblast growth and differentiation in clinical settings of assisted reproduction is warranted.
Keywords: F-actin cytoskeleton; FK506); FKBP12; [Ca2+]i; extravillous trophoblasts; inositol triphosphate receptor (IP3R); tacrolimus (TAC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Goring S.M., Levy A.R., Ghement I., Kalsekar A., Eyawo O., L’Italien G.J., Kasiske B. A network meta-analysis of the efficacy of belatacept, cyclosporine and tacrolimus for immunosuppression therapy in adult renal transplant recipients. Curr. Med. Res. Opin. 2014;30:1473–1487. doi: 10.1185/03007995.2014.898140. - DOI - PubMed
-
- Stoelinga A.E.C., Tushuizen M.E., van den Hout W.B., Girondo M., de Vries E.S., Levens A.D., Moes D.A.R., Gevers T.J.G., van der Meer S., Brouwer H.T., et al. Tacrolimus versus mycophenolate for AutoImmune hepatitis patients with incompLete response On first-line therapy (TAILOR study): A study protocol for a phase III, open-label, multicentre, randomised controlled trial. Trials. 2024;25:61. doi: 10.1186/s13063-023-07832-w. - DOI - PMC - PubMed
-
- Albaghdadi A.J.H., Kan F.W.K. Methods and Compositions for Enhancing Fertility and/or Inhibiting Pregnancy Failure and Restoring Glucose Tolerance. CA 2837359. Patent. 2011 May 31;
-
- Albaghdadi A.J.H., Hewitt M.A., Gu S.-S., Kan F.W.K. Prevention of Placental Insufficiency in the Type 2 Diabetic New-Zealand Obese Mice by Pre-Pregnancy Administration of Macrolide-Immunosuppressant Tacrolimus. Placenta. 2013;34:A83–A84. doi: 10.1016/j.placenta.2013.06.247. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

