Identification of Biochemical Determinants for Diagnosis and Prediction of Severity in 5q Spinal Muscular Atrophy Using 1H-Nuclear Magnetic Resonance Metabolic Profiling in Patient-Derived Biofluids
- PMID: 39596191
- PMCID: PMC11594255
- DOI: 10.3390/ijms252212123
Identification of Biochemical Determinants for Diagnosis and Prediction of Severity in 5q Spinal Muscular Atrophy Using 1H-Nuclear Magnetic Resonance Metabolic Profiling in Patient-Derived Biofluids
Abstract
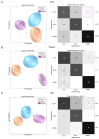
This study explores the potential of 1H-NMR spectroscopy-based metabolic profiling in various biofluids as a diagnostic and predictive modality to assess disease severity in individuals with 5q spinal muscular atrophy. A total of 213 biosamples (urine, plasma, and CSF) from 153 treatment-naïve patients with SMA across five German centers were analyzed using 1H-NMR spectroscopy. Prediction models were developed using machine learning algorithms which enabled the patients with SMA to be grouped according to disease severity. A quantitative enrichment analysis was employed to identify metabolic pathways associated with disease progression. The results demonstrate high sensitivity (84-91%) and specificity (91-94%) in distinguishing treatment-naïve patients with SMA from controls across all biofluids. The urinary and plasma profiles differentiated between early-onset (type I) and later-onset (type II/III) SMA with over 80% accuracy. Key metabolic differences involved alterations in energy and amino acid metabolism. This study suggests that 1H-NMR spectroscopy based metabolic profiling may be a promising, non-invasive tool to identify patients with SMA and for severity stratification, potentially complementing current diagnostic and prognostic strategies in SMA management.
Keywords: 1H-NMR spectroscopy; metabolic profiling; metabolomics; spinal muscular atrophy.
Conflict of interest statement
A.H., A.R., G.F.H., F.T., J.O., M.F., M.K., M.N., M.S. and S.K. have no potential conflicts of interest to declare. A.B. received honoraria for speaking at scientific meetings and serving on scientific advisory boards from Roche and Novartis, manufacturers of SMA therapeutics. A.S. and A.Z. received research funding from Biogen through a sponsored research agreement. A.Z. received consult fees, lecture fees, and travel support from Biogen, Roche, Novartis, Pfizer, and ITF Pharma. C.C. and D.P. are employees of Bruker Biospin GmbH & Co KG, Germany, a manufacturer of NMR-Spectrometers. H.K. received travel support from Biogen, Novartis, and Roche, manufacturers of therapeutics for SMA. U.S.-S. received consulting fees, lecture honoraria, and travel support from Biogen, Novartis, and Roche. H.K. received travel support from Sanofi, Novartis, Biogen, and Roche. J.J. received fees for advisory board attendance from Biogen, Novartis, and Roche. K.V. received travel support and lecture honoraria from Biogen, Novartis, and Sanofi. P.C. received lecture honoraria from Roche and Novartis and is the CEO of SMATheria Gmbh, a private non-profit research institute focusing on SMA. U.S.-S. and W.M.F. received consult fees, lecture honoraria, and travel support from Roche, Biogen, and Novartis. M.W. received consult fees and attended the advisory boards of Biogen and Roche and received travel support from Biogen. W.W. received consulting fees from Roche. A.Z. received consulting fees, honoraria, and travel support from Biogen, Novartis, and Roche.
Figures




References
-
- Lunn M.R., Wang C.H. Spinal Muscular Atrophy. Lancet Seminar Spinal Muscular Atrophy. Lancet. 2008;6736 doi: 10.1016/s0140-6736(08)60921-6. - DOI
-
- Mercuri E., Finkel R.S., Muntoni F., Wirth B., Montes J., Main M., Mazzone E.S., Vitale M., Snyder B., Quijano-Roy S., et al. Diagnosis and Management of Spinal Muscular Atrophy: Part 1: Recommendations for Diagnosis, Rehabilitation, Orthopedic and Nutritional Care. Neuromuscul. Disord. 2018;28:103–115. doi: 10.1016/j.nmd.2017.11.005. - DOI - PubMed
-
- Erdos J., Wild C. Mid- and Long-Term (at Least 12 Months) Follow-up of Patients with Spinal Muscular Atrophy (SMA) Treated with Nusinersen, Onasemnogene Abeparvovec, Risdiplam or Combination Therapies: A Systematic Review of Real-World Study Data. Eur. J. Paediatr. Neurol. 2022;39:1–10. doi: 10.1016/j.ejpn.2022.04.006. - DOI - PubMed
-
- Pechmann A., Behrens M., Dörnbrack K., Tassoni A., Wenzel F., Stein S., Vogt S., Zöller D., Bernert G., Hagenacker T., et al. Improved Upper Limb Function in Non-Ambulant Children with SMA Type 2 and 3 during Nusinersen Treatment: A Prospective 3-Years SMArtCARE Registry Study. Orphanet J. Rare Dis. 2022;17:384. doi: 10.1186/s13023-022-02547-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

