Underneath the Gut-Brain Axis in IBD-Evidence of the Non-Obvious
- PMID: 39596193
- PMCID: PMC11594934
- DOI: 10.3390/ijms252212125
Underneath the Gut-Brain Axis in IBD-Evidence of the Non-Obvious
Abstract
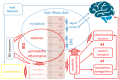
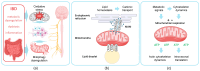
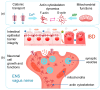
The gut-brain axis (GBA) plays a pivotal role in human health and wellness by orchestrating complex bidirectional regulation and influencing numerous critical processes within the body. Over the past decade, research has increasingly focused on the GBA in the context of inflammatory bowel disease (IBD). Beyond its well-documented effects on the GBA-enteric nervous system and vagus nerve dysregulation, and gut microbiota misbalance-IBD also leads to impairments in the metabolic and cellular functions: metabolic dysregulation, mitochondrial dysfunction, cationic transport, and cytoskeleton dysregulation. These systemic effects are currently underexplored in relation to the GBA; however, they are crucial for the nervous system cells' functioning. This review summarizes the studies on the particular mechanisms of metabolic dysregulation, mitochondrial dysfunction, cationic transport, and cytoskeleton impairments in IBD. Understanding the involvement of these processes in the GBA may help find new therapeutic targets and develop systemic approaches to improve the quality of life in IBD patients.
Keywords: IBD; cationic transport; cytoskeleton; gut–brain axis; inflammation; lipidome; metabolome; mitochondrial function.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

