Nanotechnology-Driven Delivery of Caffeine Using Ultradeformable Liposomes-Coated Hollow Mesoporous Silica Nanoparticles for Enhanced Follicular Delivery and Treatment of Androgenetic Alopecia
- PMID: 39596238
- PMCID: PMC11595114
- DOI: 10.3390/ijms252212170
Nanotechnology-Driven Delivery of Caffeine Using Ultradeformable Liposomes-Coated Hollow Mesoporous Silica Nanoparticles for Enhanced Follicular Delivery and Treatment of Androgenetic Alopecia
Abstract
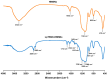
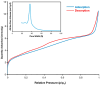
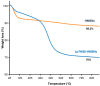
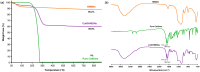
Androgenetic alopecia (AGA) is caused by the impact of dihydrotestosterone (DHT) on hair follicles, leading to progressive hair loss in men and women. In this study, we developed caffeine-loaded hollow mesoporous silica nanoparticles coated with ultradeformable liposomes (ULp-Caf@HMSNs) to enhance caffeine delivery to hair follicles. Caffeine, known to inhibit DHT formation, faces challenges in skin penetration due to its hydrophilic nature. We investigated caffeine encapsulated in liposomes, hollow mesoporous silica nanoparticles (HMSNs), and ultradeformable liposome-coated HMSNs to optimize drug delivery and release. For ultradeformable liposomes (ULs), the amount of polysorbate 20 and polysorbate 80 was varied. TEM images confirmed the mesoporous shell and hollow core structure of HMSNs, with a shell thickness of 25-35 nm and a hollow space of 80-100 nm. SEM and TEM analysis showed particle sizes ranging from 140-160 nm. Thermal stability tests showed that HMSNs coated with ULs exhibited a Td10 value of 325 °C and 70% residue ash, indicating good thermal stability. Caffeine release experiments indicated that the highest release occurred in caffeine-loaded HMSNs without a liposome coating. In contrast, systems incorporating ULp-Caf@HMSNs exhibited slower release rates, attributable to the dual encapsulation mechanism. Confocal laser scanning microscopy revealed that ULs-coated particles penetrated deeper into the skin than non-liposome particles. MTT assays confirmed the non-cytotoxicity of all HMSN concentrations to human follicle dermal papilla cells (HFDPCs). ULp-Caf@HMSNs promoted better cell viability than pure caffeine or caffeine-loaded HMSNs, highlighting enhanced biocompatibility without increased toxicity. Additionally, ULp-Caf@HMSNs effectively reduced ROS levels in DHT-damaged HFDPCs, suggesting they are promising alternatives to minoxidil for promoting hair follicle growth and reducing hair loss without increasing oxidative stress. This system shows promise for treating AGA.
Keywords: androgenetic alopecia; caffeine; confocal laser scanning microscopy (CLSM); follicular delivery; hair follicle dermal papilla cells (HFDPCs); hollow mesoporous silica nanoparticles (HMSNs); ultradeformable liposomes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Kaur K., Kaur J., Sharma S. Evaluation of trichoscopic findings in androgenetic alopecia and their association with disease severity. Iran. J. Dermatol. 2022;25:117–122.
-
- Khantham C., Ruksiriwanich W., Sringarm K., Prom-U-Thai C., Jamjod S., Arjin C., Muangsanguan A., Rachtanapun P., Jantanasakulwong K., Phimolsiripol Y. Effects of bioactive composition in Oryza sativa L. cv. KDML105 bran extract on gene expression related to hair cycle in human hair follicle dermal papilla cells. Agronomy. 2023;13:295. doi: 10.3390/agronomy13020295. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

