Cysteine Conjugation: An Approach to Obtain Polymers with Enhanced Muco- and Tissue Adhesion
- PMID: 39596243
- PMCID: PMC11594736
- DOI: 10.3390/ijms252212177
Cysteine Conjugation: An Approach to Obtain Polymers with Enhanced Muco- and Tissue Adhesion
Abstract
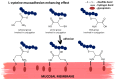
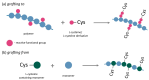
The modification of polymers towards increasing their biocompatibility gathers the attention of scientists worldwide. Several strategies are used in this field, among which chemical post-polymerization modification has recently been the most explored. Particular attention revolves around polymer-L-cysteine (Cys) conjugates. Cys, a natural amino acid, contains reactive thiol, amine, and carboxyl moieties, allowing hydrogen bond formation and improved tissue adhesion when conjugated to polymers. Conjugation of Cys and its derivatives to polymers has been examined mostly for hyaluronic acid, chitosan, alginate, polyesters, polyurethanes, poly(ethylene glycol), poly(acrylic acid), polycarbophil, and carboxymethyl cellulose. It was shown that the conjugation of Cys and its derivatives to polymers significantly increased their tissue adhesion, particularly mucoadhesion, stability at physiological pH, drug encapsulation efficiency, drug release, and drug permeation. Conjugates were also non-toxic toward various cell lines. These properties make Cys conjugation a promising strategy for advancing polymer applications in drug delivery systems and tissue engineering. This review aims to provide an overview of these features and to present the conjugation of Cys and its derivatives as a modern and promising approach for enhancing polymer tissue adhesion and its application in the medical field.
Keywords: L-cysteine; amino acid conjugation; mucoadhesion; polymer modification; polymer-L-cysteine conjugates; tissue adhesion.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Screening of anionic-modified polymers in terms of stability, disintegration, and swelling behavior.Drug Dev Ind Pharm. 2017 Nov;43(11):1866-1872. doi: 10.1080/03639045.2017.1349789. Epub 2017 Jul 18. Drug Dev Ind Pharm. 2017. PMID: 28673094
-
Mucoadhesive and cohesive properties of poly(acrylic acid)-cysteine conjugates with regard to their molecular mass.Eur J Pharm Sci. 2003 Jan;18(1):89-96. doi: 10.1016/s0928-0987(02)00245-2. Eur J Pharm Sci. 2003. PMID: 12554077
-
Novel bioadhesive polymers as intra-articular agents: Chondroitin sulfate-cysteine conjugates.Eur J Pharm Biopharm. 2016 Apr;101:25-32. doi: 10.1016/j.ejpb.2016.01.006. Epub 2016 Jan 22. Eur J Pharm Biopharm. 2016. PMID: 26807491
-
'Smart' delivery systems for biomolecular therapeutics.Orthod Craniofac Res. 2005 Aug;8(3):219-25. doi: 10.1111/j.1601-6343.2005.00336.x. Orthod Craniofac Res. 2005. PMID: 16022724 Review.
-
Polymer-based nanoparticles for oral insulin delivery: Revisited approaches.Biotechnol Adv. 2015 Nov 1;33(6 Pt 3):1342-54. doi: 10.1016/j.biotechadv.2015.02.010. Epub 2015 Feb 26. Biotechnol Adv. 2015. PMID: 25728065 Review.
Cited by
-
Synthetic and semi-synthetic antioxidants in medicine and food industry: a review.Front Pharmacol. 2025 Jul 22;16:1599816. doi: 10.3389/fphar.2025.1599816. eCollection 2025. Front Pharmacol. 2025. PMID: 40766767 Free PMC article. Review.
References
-
- Vert M. Polymeric Biomaterials: Strategies of the Past vs. Strategies of the Future. Prog. Polym. Sci. 2007;32:755–761. doi: 10.1016/j.progpolymsci.2007.05.006. - DOI
-
- Maitz M.F. Applications of Synthetic Polymers in Clinical Medicine. Biosurface Biotribology. 2015;1:161–176. doi: 10.1016/j.bsbt.2015.08.002. - DOI
-
- Nazeer N., Ahmed M. Polymers in Medicine. In: Narain R., editor. Polymer Science and Nanotechnology. Elsevier; Amsterdam, The Netherlands: 2020. pp. 281–323. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

