The Multifaceted Ubiquitination of BIK1 During Plant Immunity in Arabidopsis thaliana
- PMID: 39596247
- PMCID: PMC11594851
- DOI: 10.3390/ijms252212187
The Multifaceted Ubiquitination of BIK1 During Plant Immunity in Arabidopsis thaliana
Abstract
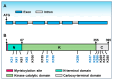
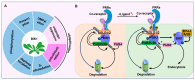
As sessile organisms, the plant immune system plays a vital role in protecting plants from the widespread pathogens in the environment. The Arabidopsis thaliana (Arabidopsis) receptor-like cytoplasmic kinase BOTRYTIS-INDUCED KINASE1 (BIK1) acts as a central regulator during plant immunity. As such, not only the BIK1 protein accumulation but also the attenuation is tightly regulated to ensure effective immune responses. Recent studies have highlighted the critical roles of ubiquitination in maintaining BIK1 homeostasis. Here, we review the latest advances in the ubiquitination of BIK1 in plant immunity, which is mediated by ubiquitin ligases PUB25/26, RHA3A/B, RGLG1/2, and PUB4. Additionally, we summarize and discuss the sites and types of BIK1 ubiquitination. Collectively, these analyses not only illustrate that the differential modifications on BIK1 by multiple ubiquitin ligases hold a crucial position in plant immunity but also provide a good example for future studies on ubiquitin-mediated modifications in plants.
Keywords: Arabidopsis thaliana; BIK1; plant immunity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

