Prenatal Acoustic Signals Influence Nestling Heat Shock Protein Response to Heat and Heterophil-to-Lymphocyte Ratio in a Desert Bird
- PMID: 39596260
- PMCID: PMC11595141
- DOI: 10.3390/ijms252212194
Prenatal Acoustic Signals Influence Nestling Heat Shock Protein Response to Heat and Heterophil-to-Lymphocyte Ratio in a Desert Bird
Abstract
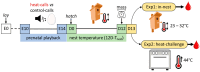
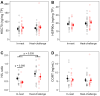
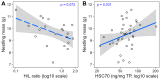
Heat shock proteins (HSPs) are essential to cellular protection against heat stress. However, the causes of inter-individual variation in HSP regulation remain unclear. This study aimed to test the impact of early-life conditions on the HSP response to heat in zebra finches. In this arid-adapted bird, incubating parents emit "heat-calls" at high temperatures, which adaptively alter offspring's phenotypes. Embryos were exposed to heat-calls or control-calls, and at 13 days post-hatch nestlings were separated into two different experiments to test responses to either chronic nest temperature ("in-nest" experiment) or an acute "heat-challenge". Blood samples were collected to measure levels of heat shock cognate 70, heat shock protein 90α, corticosterone and the heterophil-to-lymphocyte (H/L) ratio. In the in-nest experiment, both HSPs were upregulated in response to increasing nest temperatures only in control-calls nestlings (HSC70: p = 0.010, HSP90α: p = 0.050), which also had a marginally higher H/L ratio overall than heat-call birds (p = 0.066). These results point to a higher heat sensitivity in control-call nestlings. Furthermore, comparing across experiments, only the H/L ratio differed, being higher in heat-challenged than in in-nest nestlings (p = 0.009). Overall, this study shows for the first time that a prenatal acoustic signal of heat affects the nestling HSP response to postnatal temperature.
Keywords: Taeniopygia guttata; acoustic developmental programming; glucocorticoid; leucocyte profile; phenotypic plasticity; prenatal sound; stress proteins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Romero L.M., Wingfield J.C. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford University Press; New York, NY, USA: 2015. (Oxford Series in Behavioral Neuroendocrinology).
-
- Sørensen J.G. Application of Heat Shock Protein Expression for Detecting Natural Adaptation and Exposure to Stress in Natural Populations. Curr. Zool. 2010;56:703–713. doi: 10.1093/czoolo/56.6.703. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

