Acute and Chronic Resistance Training, Acute Endurance Exercise, nor Physiologically Plausible Lactate In Vitro Affect Skeletal Muscle Lactylation
- PMID: 39596281
- PMCID: PMC11594461
- DOI: 10.3390/ijms252212216
Acute and Chronic Resistance Training, Acute Endurance Exercise, nor Physiologically Plausible Lactate In Vitro Affect Skeletal Muscle Lactylation
Abstract
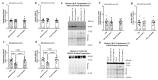
We examined changes in skeletal muscle protein lactylation and acetylation in response to acute resistance exercise, chronic resistance training (RT), and a single endurance cycling bout. Additionally, we performed in vitro experiments to determine if different sodium lactate treatments affect myotube protein lactylation and acetylation. The acute and chronic RT study (12 college-aged participants) consisted of 10 weeks of unilateral leg extensor RT with vastus lateralis (VL) biopsies taken at baseline, 24 h following the first RT bout, and the morning of the last day of the RT bout. For the acute cycling study (9 college-aged participants), VL biopsies were obtained before, 2 h after, and 8 h after 60 min of cycling. For in vitro experiments, C2C12 myotubes were treated with varying levels of sodium lactate, including LOW (1 mM for 24 h), HIGH (10 mM for 24 h), and PULSE (10 mM for 30 min followed by 1 mM for 23.5-h). Neither acute nor chronic RT significantly affected nuclear or cytoplasmic protein lactylation. However, cytoplasmic protein acetylation was significantly reduced following one RT bout (-15%, p = 0.002) and chronic RT (-16%, p = 0.006). Cycling did not acutely alter post-exercise global protein lactylation or acetylation patterns. Lastly, varying 24 h lactate treatments did not alter nuclear or cytoplasmic protein lactylation or acetylation, cytoplasmic protein synthesis levels, or myotube diameters. These findings continue to support the idea that exercise induces more dynamic changes in skeletal muscle protein acetylation, but not lactylation. However, further human research with more sampling timepoints and a lactylomics approach are needed to determine if, at all, different exercise modalities affect skeletal muscle protein lactylation.
Keywords: hypertrophy; protein acetylation; protein lactylation; skeletal muscle.
Conflict of interest statement
None of the authors have conflicts of interest to report in relation to this work. In the interest of full disclosure, M.D.R. and A.D.F. report current research contracts and gifts from several industry sponsors. M.D.R. and A.D.F act as consultants for industry entities and healthcare entities, respectively, in accordance with rules established by Auburn University’s Conflict of Interest (COI) Policies.
Figures




References
-
- Roberts M.D., McCarthy J.J., Hornberger T.A., Phillips S.M., Mackey A.L., Nader G.A., Boppart M.D., Kavazis A.N., Reidy P.T., Ogasawara R., et al. Mechanisms of mechanical overload-induced skeletal muscle hypertrophy: Current understanding and future directions. Physiol. Rev. 2023;103:2679–2757. doi: 10.1152/physrev.00039.2022. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

