Erucin, a Natural Isothiocyanate, Prevents Polyglutamine-Induced Toxicity in Caenorhabditis elegans via aak-2/AMPK and daf-16/FOXO Signaling
- PMID: 39596283
- PMCID: PMC11594550
- DOI: 10.3390/ijms252212220
Erucin, a Natural Isothiocyanate, Prevents Polyglutamine-Induced Toxicity in Caenorhabditis elegans via aak-2/AMPK and daf-16/FOXO Signaling
Abstract
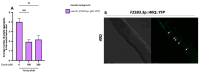
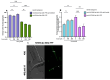
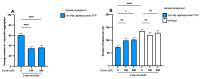
Several neurodegenerative diseases (NDDs), such as Huntington's disease, six of the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinobulbar muscular atrophy, are caused by abnormally long polyglutamine (polyQ) tracts. Natural compounds capable of alleviating polyQ-induced toxicity are currently of great interest. In this work, we investigated the modulatory effect against polyQ neurotoxic aggregates exerted by erucin (ERN), an isothiocyanate naturally present in its precursor glucoerucin in rocket salad leaves and in its oxidized form, sulforaphane (SFN), in broccoli. Using C. elegans models expressing polyQ in different tissues, we demonstrated that ERN protects against polyQ-induced toxicity and that its action depends on the catalytic subunit of AMP-activated protein kinase (aak-2/AMPKα2) and, downstream in this pathway, on the daf-16/FOXO transcription factor, since nematodes deficient in aak-2/AMPKα2 and daf-16 did not respond to the treatment, respectively. Although triggered by a different source of neurotoxicity than polyQ diseases, i.e., by α-synuclein (α-syn) aggregates, Parkinson's disease (PD) was also considered in our study. Our results showed that ERN reduces α-syn aggregates and slightly improves the motility of worms. Therefore, further preclinical studies in mouse models of protein aggregation are justified and could provide insights into testing whether ERN could be a potential neuroprotective compound in humans.
Keywords: AMPK; Caenorhabditis elegans; daf-16/FOXO; erucin; neuroprotection; polyQ toxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Caldero-Escudero E., Romero-Sanz S., De la Fuente S. Using C. elegans as a model for neurodegenerative diseases: Methodology and evaluation. Methods Cell Biol. 2024;188:1–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

